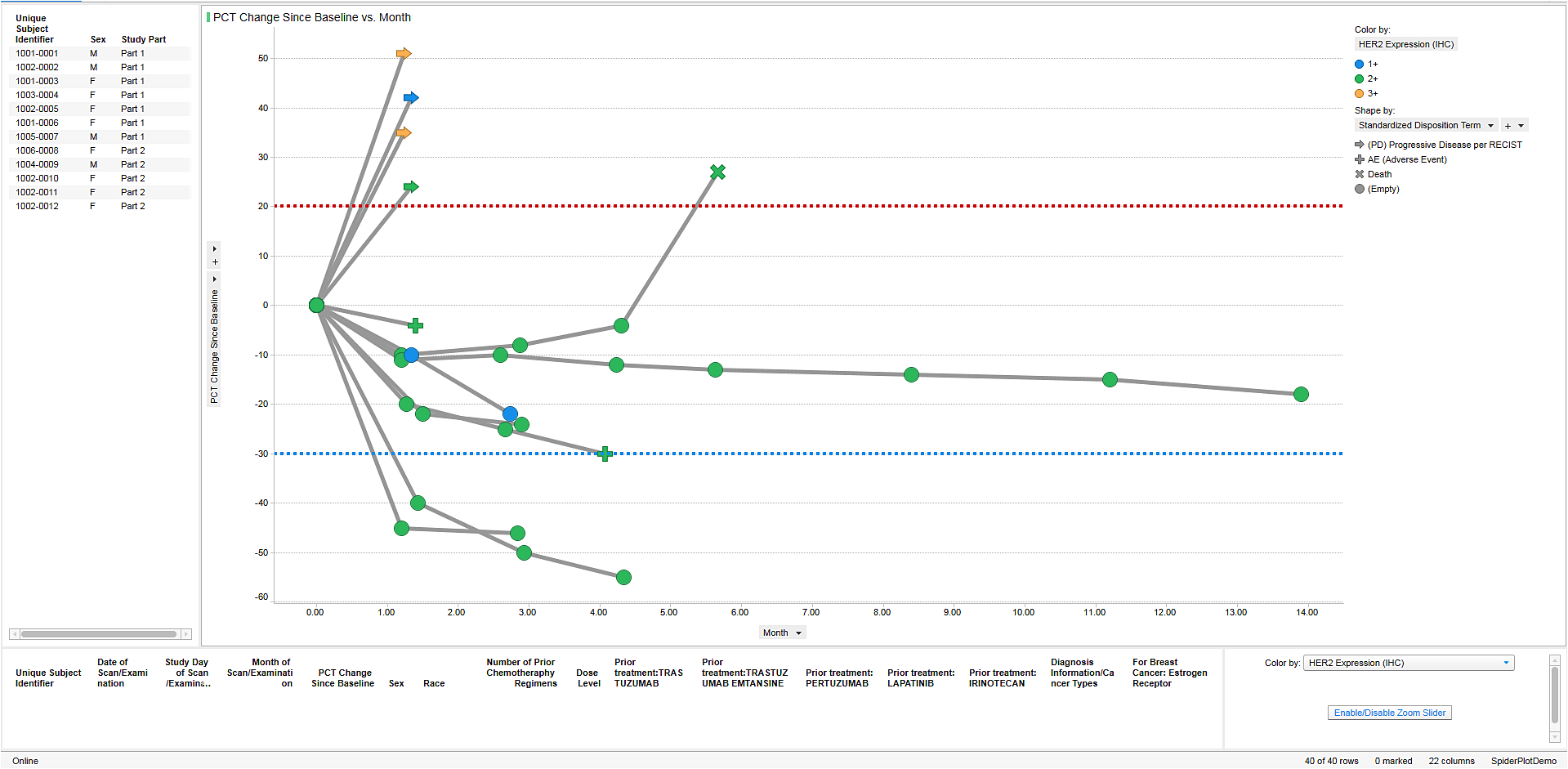
This webcast discusses how drug safety teams can use dynamic visual analytics to uncover safety issues earlier. All the while, complying with extremely complex and ever-evolving regulatory requirements. We will also provide recommendations for advanced analytics on real-world data and active surveillance for a proactive PV platform.
In this webcast, attendees will learn:
- Essential analytics and visualization capabilities to streamline case analysis; save up to three weeks on reporting time
- How to leverage “real time” data insights to ensure regulatory reporting compliance
- How real-world data and active surveillance can transform your safety surveillance program
This is the third in the series Four Steps to Shorten Your Clinical Trials with Informatics.This series explores proven ways to develop go/no-go insights faster so clinical development teams can:
- Get submission-ready faster
- Reduce the risk of a rejected submission or potential market withdrawal
- Optimize data provisioning, aggregation and review
Speakers

Masha Hoffey, Director of Clinical Analytics, PerkinElmer Informatics
Masha Hoffey is the Director of Clinical Analytics at PerkinElmer. She has over eight years of experience with clinical and regulatory applications. She oversees the Clinical Practice, including product development and services. She also served as Head of Services and Support at Virtify, Inc. and was part of the business analysis teams at Merge Healthcare and PAREXEL International, driving clinical and regulatory metrics/reporting strategy and leading customer implementations. Ms. Hoffey holds a BA from Williams College and an MS in Regulatory Affairs for Drugs, Devices, and Biologics from Northeastern University.
Connect on LinkedIn: https://www.linkedin.com/in/masha-hoffey-a7814740

James (Jamie) Powers, Director of Real World Evidence and Data Science, PerkinElmer Informatics
Jamie Powers is the Director of Real World Evidence and Data Science at PerkinElmer Informatics. With over a decade of experience as a Data Scientist and Statistician, Jamie seeks to help Biopharma companies realize the value of predictive analytics and machine learning, targeting tangible business outcomes. His workshops and conference presentations are popular for their approachable introduction to technical topics and business use cases in pharmaceutical drug development. Jamie holds a doctoral degree in Biostatistics from the University of North Carolina at Chapel Hill.
Connect on LinkedIn: https://www.linkedin.com/in/jamiepowersdrph/
Who Should Attend?
Clinical development and clinical operation teams from:
- Pharmaceutical and Biotechnology companies
- Medical Device manufacturers
- Clinical Research Organizations (CROs)
- Academic Research Organizations (AROs)
Xtalks Partner
Perkin Elmer
PerkinElmer’s advanced analytics and services solutions for Clinical Development help the world’s leading biopharmaceutical, medical device and diagnostics manufactures discover new therapeutics faster by streamlining clinical operations, transforming risk into safety and enabling actionable decisions that can lead to better health outcomes.
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account