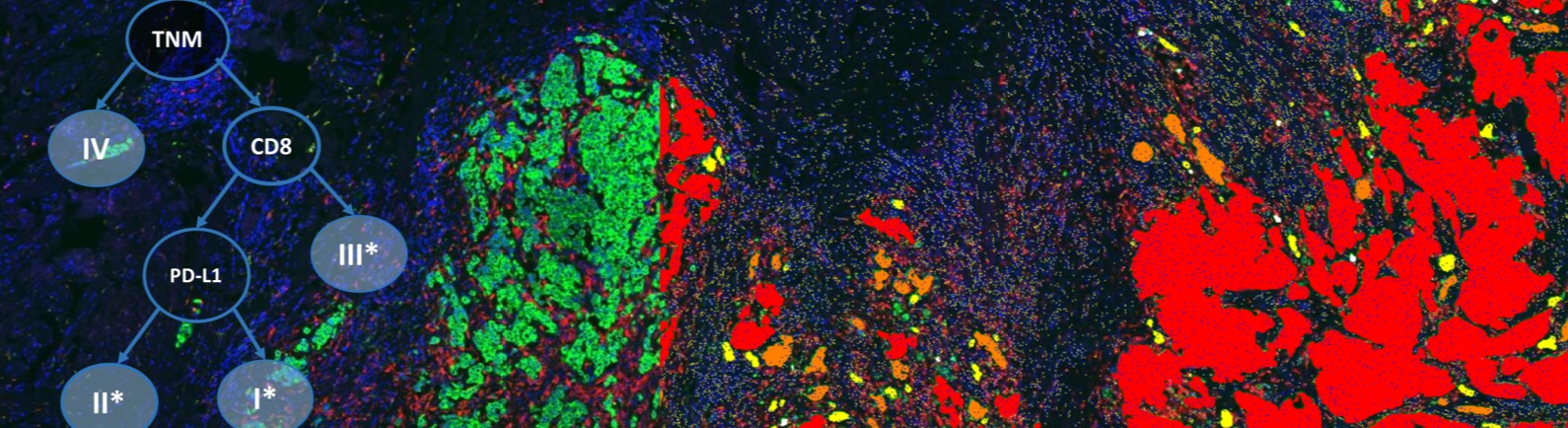
Traditional immunohistochemistry (IHC) assays are one of the most commonly used diagnostic assays because they are easily deployed and cost effective. However, IHC assays do pose significant challenges to companion diagnostic and patient stratification use in regards to their lack of standardization and reliability as well as producing qualitative, and not quantitative, results. Due to these challenges, there has been limited success of an IHC assay becoming a companion diagnostic. However, IHC assays have great promise as a powerful and robust diagnostic tool that could be used to stratify patients for clinical trials and ultimately demonstrate the medical value of important cancer therapies.
This webinar will go into detail on how to devise a method of quantitative IHC assay design that overcomes the inherent challenges of traditional IHC assays. With quantitative IHC assays, clinical researchers can increase the chance of finding the optimal cutpoint of biomarker expression and maximizing the chance of success for the IHC assay in companion diagnostic use. You will learn how, through use of standard curves and quantitative image analysis, the required target expression level can be determined and IHC assays re-optimized for more uniform and consistent scoring by a pathologist.
In this webinar, Dr. Moulis will walk you through:
- How quantitative image analysis can impact and inform IHC assay design to increase standardization – from optimizing the staining protocols to overcoming inconsistent tissue collection practices.
- How quantitative IHC assay scoring allows for comparison of measurements across nonparallel staining runs, identifying and monitoring of sources of variability in the assay, as well as identifying mechanistically relevant cutpoints.
- How quantitative IHC assays can undergo rigorous analytic validation to produce a robust IVD package that more clearly defines the assay’s limitations in order to improve patient stratification in clinical trials.
- An ideal process for designing a quantitative IHC assay where a candidate biomarker is selected, but the appropriate cutoff is currently unknown.
Speaker

Sharon Moulis, PhD, Director, Tissue Diagnostics Alliances, Definiens
Sharon Moulis received her PhD from Yale University, studying with Dr. David L. Rimm. Her background includes development of quantitative immunohistochemistry methods, assay design, companion diagnostic strategy and implementation, and clinical trial sample management for companion diagnostics.
Who Should Attend?
This webinar will be ideal for professionals in the Medical, Pharmaceutical, Biotech and Diagnostics fields, specifically:
- Directors and VPs of therapeutic areas
- Chief Scientific Officers
- Medical directors and officers
- Lab & Study directors
- Executive officers
Xtalks Partner
Definiens
Definiens is the leading provider of image analysis and data mining solutions for tissue diagnostics and clinical digital pathology. Definiens technology provides detailed cell-by-cell readouts from target structures on tissue slides and allows the correlation of this information with data derived from other sources, generating new knowledge and supporting better decisions in research, diagnostics and therapy. Definiens’ Tissue Phenomics approach was awarded the 2013 Frost and Sullivan Company of the Year Award for Global Tissue Diagnostics and Pathology Imaging. For more information, please visit: http://www.definiens.com/.
Media Partner
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account