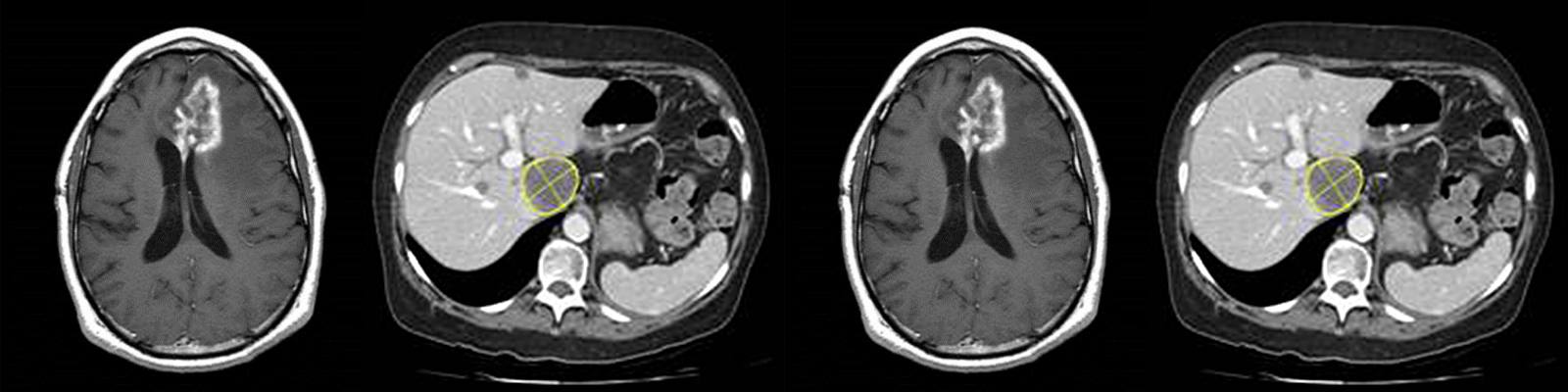
Fatty liver disease is a progressive disease that begins with accumulation of fat in the liver. This accumulation of fat in the liver can then lead to inflammation, at which point, the disease is referred to as steatohepatitis. The inflammation can then lead to fibrosis, which, when it becomes severe enough, becomes cirrhosis and may even lead to hepatocellular carcinoma. Fatty liver disease may be caused by excessive alcohol consumption. Nonalcoholic Fatty Liver Disease (NAFLD) is caused a variety of factors including poor diet and exercise (the western diet) and is the leading cause of chronic liver disease in children and adults in the United States. It is also an increasingly-common reason for liver transplants in the United States. Nonalcoholic Steatohepatitis or NASH is the progression of NAFLD from simple steatosis (fat in the liver) to steatohepatitis (fat, inflammation and tissue damage in the liver).
Lifestyle modification and bariatric surgery may be used to treat NAFLD or NASH. Drug therapy is also in development. The current standard for determining the stage and progression of the disease is histopathology from a liver biopsy. From the biopsy, a pathologist can determine the degree of steatosis, the amount of inflammation, the degree of fibrosis, and the amount of cellular damage. These four measurements currently comprise the NASH Clinical Research Network’s scoring system for the disease. There are several issues with biopsies: they are very invasive, they have a small morbidity and mortality rate, and they only sample about 0.002% of the total liver volume.
Because of the risks and sampling variability associated with liver biopsies, it would be ideal to develop noninvasive ways to assess the stage and progression of the disease. One noninvasive way to assess the majority of the measurements obtained from a biopsy is through imaging.
Join BioTelemetry Research’s Jonathan Riek, PhD, Vice President, Musculoskeletal and Metabolic Imaging and Dr. Richard Ehman, MD from the Mayo Clinic as they discuss noninvasive ways to assess NASH using imaging, including:
- Proton density fat fraction (PDFF) – a magnetic resonance imaging (MRI) technique that can accurately determine the amount of fat in the liver (steatosis)
- T1 relaxometry– an MRI technique that measures the amount of extracellular fluid (which is related to the amount of fibrosis and inflammation)
- Magnetic resonance elastography (MRE) – a technique that builds upon MRI to determine the stiffness of the liver, which can be used to determine the degree of fibrosis
- The latest advances in MRE and how they can be used to assess inflammation
Register today to discover how partnering with BioTelemetry Research’s experts will optimize your liver clinical trial!
This webinar will discuss noninvasive ways to assess NASH using imaging, including proton density fat fraction (PDFF), T1 relaxometry, Magnetic resonance elastography (MRE) and the latest advances in MRE and how they can be used to assess inflammation
Speakers

Jonathan Riek, Vice President, Musculoskeletal and Metabolic Imaging, BioTelemetry Research (Cardiocore & VirtualScopics)
Jonathan Riek, Ph.D., serves as the Vice President of Musculoskeletal & Metabolic Imaging for BioTelemetry Research. He has a PhD in Electrical Engineering from the University of Rochester, and his doctoral thesis was on motion and artifact reduction in MRI. He worked on the development of a 3D CT scanner as a postdoctoral researcher, and then spent 7 years in the research labs at the Eastman Kodak Company. While at Kodak, Jonathan worked in the areas of motion estimation, frame rate conversion and video compression. He also served as the liaison between the image science division and the corporate software group. He joined VirtualScopics in 2000 to return to medical imaging and apply his skills interfacing between image science and software. In 2004, he led the development of the system that VirtualScopics still uses to run clinical trials and analyze medical images. In his current role, Jonathan has scientific responsibility for all of Biotelemetry Research’s musculoskeletal and metabolic imaging studies, including arthritis, muscle diseases, diabetes and liver diseases such as NASH and NAFLD. He holds four patents and has published many articles on various topics in MRI.

Richard L. Ehman, M.D., Professor of Radiology, Blanche R. & Richard J. Erlanger Professor of Medical Research, Mayo Clinic; President and CEO, Resoundant, Inc.
Richard L. Ehman, M.D., is Professor of Radiology at the Mayo Clinic and Emeritus member of the Mayo Clinic Board of Trustees. His research program is focused on developing new imaging technologies. He holds more than 40 patents and many of these inventions are widely used in medical care.
He has served as chair of the Radiology and Nuclear Medicine Study Section of the National Institutes of Health (NIH), as a member of the Advisory Council of the NationalInstitute of Biomedical Imaging and Bioengineering of the NIH, and as a member of the Council of Councils of the NIH. Dr. Ehman was awarded the Gold Medal of the International Society for Magnetic Resonance in Medicine in 1995 for his research contributions and the Outstanding Researcher Award of the Radiological Society of North America in 2006. He was elected to the Institute of Medicine of the National Academies of Science in 2010 and named Mayo Clinic Distinguished Investigator in 2014. He was awarded the Gold Medal of the Asian Oceanian Society of Radiology in 2016.
Dr. Ehman has served as president of many professional organizations, including the International Society for Magnetic Resonance in Medicine in 2002-03, the Academy of Radiology Research in 2012-14, and the Society for Body Computed Tomography and Magnetic Resonance in 2013-14. He currently serves as President of the Radiological Society of North America (RSNA).
Who Should Attend?
This webinar will benefit medical and non-medical professionals in the biopharmaceutical industry, especially those supporting liver drug development with roles in:
- Clinical Research
- Clinical Development
- Medical Affairs
- Clinical Operations
- Project Management
- Regulatory Affairs
- Procurement
- Outsourcing
Xtalks Partner
Biotelemetry
As a division of BioTelemetry, Inc. (NASDAQ: BEAT), BioTelemetry Research provides expert Cardiac and Imaging core lab solutions for the advancement of clinical drug development. Our cardiac network processes over 2 billion heartbeats a day, while supporting over 20,000 sites and 30,000 devices monthly, and monitoring nearly 600,000 patients and research subjects a year. We offer global operational support for cardiovascular monitoring in all therapeutic areas, and advanced imaging analyses in cardiovascular, oncology, neurology, metabolic, musculoskeletal and medical device studies. Our research team is comprised of key opinion leaders, board certified radiologists and cardiologists, sub-specialty scientists, and highly trained technicians. These experts acquire, evaluate, and report high-quality data through an efficient, cloud-based infrastructure. Additionally, we offer integrated spirometry and ECG services in cardio-respiratory clinical trials through an exclusive alliance with Vitalograph. For more information please visit www.gobio.com/research.
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account