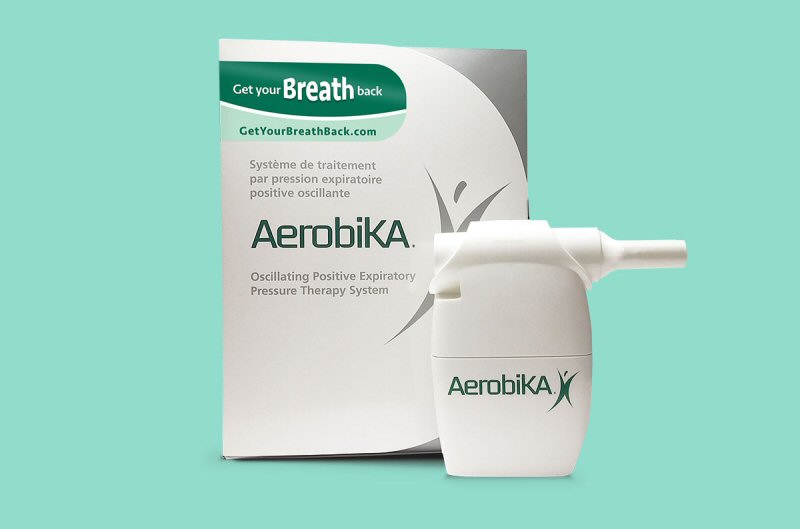
Trudell Medical International’s Aerobika oscillating positive expiratory pressure (OPEP) device can reduce the risk of post-operative pulmonary complications in patients, according to real-world research published in the journal Pulmonary Therapy. In all, 288 patients were included in the retrospective study which found that patients treated using the standard of care – including incentive spirometry – along with the Aerobika device were associated with less re-hospitalizations, faster patient discharge and lower costs.
About 14 percent of patients using the Aerobika OPEP device required re-hospitalization 30 days after they were originally discharged, compared with 23 percent of those in the standard of care group. What’s more, hospitalization costs associated with caring for these patients were 80 percent lower than those for the group not given the Aerobika device.
Patients undergoing cardiac, thoracic or upper abdominal surgery face a significant risk of developing post-operative pulmonary complications. These complications have been associated with a 10 to 20 percent higher rate of death, and billions of dollars in excess healthcare spending.
RELATED: Medical Device Reduces Need for Medication to Treat COPD
Physical therapy to increase lung functioning and reduce complications after surgery often includes incentive spirometry, which involves the use of a ball or piston which is raised when a patient inhales through the device. While it’s thought that this helps patients take slow, steady breaths, clinical evidence has not necessarily supported its use.
In contrast, the drug-free Aerobika device using oscillating positive expiratory pressure when a patient exhales through the device which expands lung capacity and allows for better expulsion of mucus. Leaning on clinical data from both controlled trials and real-world evidence of improved outcomes in patients with chronic obstructive pulmonary disorder (COPD), the makers of the Aerobika device sought to study its usefulness in preventing post-operative pulmonary complications.
“Based on the study findings and taking into consideration both the low cost and low risk safety profile of the Aerobika OPEP device, I would suggest that it could be beneficial to include as standard of care in all such post-operative patients,” said co-author Dr. Jason Suggett. “The fact that patients in the Aerobika OPEP device group in our study incurred lower healthcare costs in the 30-day period following discharge (mainly as a result of fewer complications requiring readmission) is particularly relevant as providers and insurers look to reduce early rehospitalization.”







Join or login to leave a comment
JOIN LOGIN