A Washington-based company, Sixty Degrees Pharmaceuticals, has begun the distribution of a new anti-malarial drug, Arakoda (tafenoquine) into the US healthcare marketplace. This is a drug that has been approved for the prevention of malaria in adults. This is the first anti-malaria product that the US Food and Drug Administration (FDA) has approved in over 18 years.
Sixty Degrees Pharmaceuticals’ mission since 2010 has been to find a new way to improve lives by developing and distributing new medicines for the treatment and prevention of tropical disease. These diseases include malaria and dengue infections.
“Malaria is one of the most malicious diseases, on the rise in both US and other parts of the world,” said Geoffrey Dow, chief executive officer of Sixty Degree Pharmaceuticals.
According to the World Health Organization, a child dies every two minutes from malaria. There are more than 200 million new cases of the disease reported every year.
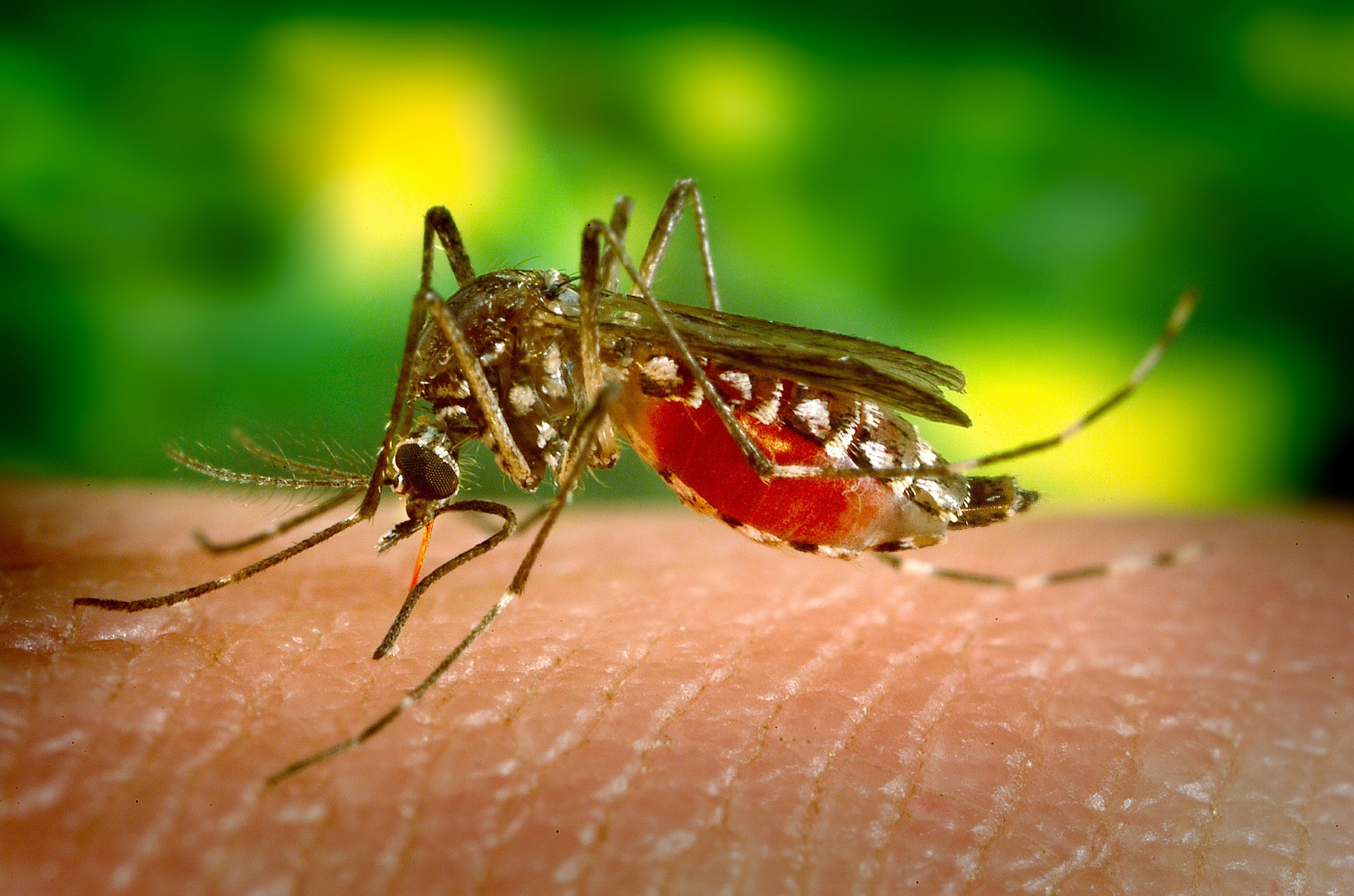
Malaria is caused when parasites are transmitted to people through the bite of an infected mosquito. It is caused by the Plasmodium parasite and is spread through the bite of an infected Anopheles mosquito vector. One of the most deadly of parasites is the P. falciparum. The symptoms of infection include fever, headache and chills which begin to show 10 to 15 days after a mosquito bite. If left untreated, the development of P. falciparum can cause severe illness and death.
Arakoda was first shipped to the US army in September 2019. The product is now available commercially through select retail pharmacy outlets and wholesalers. Arakoda is a drug containing the active ingredient tafenoquine. According to the company’s press release, “aminoquinoline is chemically derived from Primaquine, with activity against all types of malaria. It was first synthesized by scientists at WRAIR in 1978.”
“It [malaria] poses a significant risk to millions of healthy individuals traveling to areas where malaria is endemic, including those traveling for leisure, employees of non-governmental organizations, industrial and business workers, and military forces,” said Dow.
The product is supplied in 100mg tablets and taken orally. An initial dose is taken before travel and is then intended to be taken once a week in high threat areas.








Join or login to leave a comment
JOIN LOGIN