Alzheimer’s disease researchers have long known that a variant of the gene encoding apolipoprotein – known as ApoE4 – is one of the most important genetic risk factors for the neurodegenerative disease. Now, new research out of the Gladstone Institute of Neurological Disease in California has identified key species differences in the role of ApoE4 in mice and human neurons, and a potential mechanism by which its neurotoxic effects could be blocked.
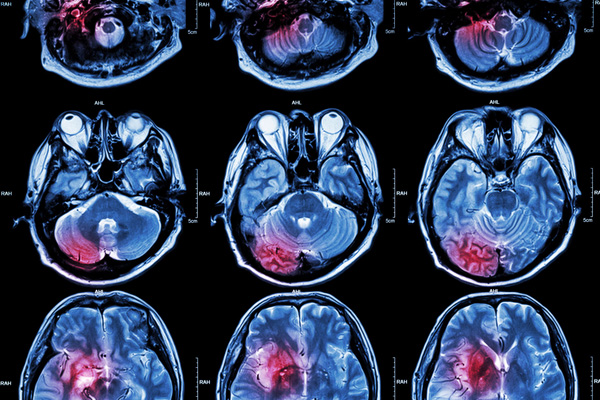
Various studies have reported that individuals who carry one copy of the ApoE4 gene variant have a two- or three-fold higher risk of developing late-onset Alzheimer’s disease, while those who are homozygous for the gene variant are 12 times more likely to develop this type of dementia. People with the ApoE4 gene variant have a higher risk of accumulating amyloid-beta and tau proteins in the brain, which is pathology characteristic of Alzheimer’s disease.
Still, researchers have been unsure as to why the E4 variant of Apo – whose normal role is to regulate cholesterol levels through the synthesis of lipoproteins – has such neurotoxic effects. Some hypotheses have been that ApoE4 may have diminished function compared to the more-common ApoE3, or the gene variant may even have gained neurotoxic effects.
“It’s fundamentally important to address this question because it changes how you treat the problem,” Dr. Yadong Huang, a professor of neurology and pathology at the University of California, San Francisco, told MedicalNewsToday. “If the damage is caused due to the loss of a protein’s function, you would want to increase protein levels to supplement those functions. But if the accumulation of a protein leads to a toxic function, you want to lower production of the protein to block its detrimental effect.”
Instead of just improving their understanding of the role of ApoE4, Huang and his colleagues sought to address a fundamental issue of Alzheimer’s research today: the lack of preclinical models that can accurately predict the human patient’s response to a therapy. In the past 20 years, the vast majority of candidates designed to treat Alzheimer’s disease have shown promising results in animal models but failed to show efficacy in human trials.
RELATED: Claims That Ibuprofen Could Prevent Alzheimer’s Disease Are Met with Controversy
Most recently, North Carolina-based vTv Therapeutics announced their investigational Alzheimer’s drug azeliragon failed to improve cognitive or functional outcomes in patients with the neurodegenerative disease in a Phase III clinical trial. The company is now discontinuing their clinical development of the drug for an Alzheimer’s indication but hopes to identify another therapeutic use for their candidate.
“Many drugs work beautifully in a mouse model, but so far they’ve all failed in clinical trials,” said Huang. “One concern within the field has been how poorly these mouse models really mimic human disease.”
Using skin cells collected from patients with, and without, the Apo4 gene variant, Huang and his research team generated pluripotent stem cells capable of then being differentiated into neurons. The ApoE4 variant showed an altered structure compared to ApoE3, which impeded its ability to properly function in the neurons. Interestingly, while the ApoE4 gene variant was associated with increased tau phosphorylation and amyloid-beta production in the human neurons, the same was not observed in comparable mouse neurons.
“There’s an important species difference in the effect of Apoe4 on amyloid beta,” said Chengzhong Wang, a research scientist in the Department of Neurology at the University of California and first author on the study published in Nature Medicine. “Increased amyloid beta production is not seen in mouse neurons and could potentially explain some of the discrepancies between mice and humans regarding drug efficacy. This will be very important information for future drug development.”
The team took their research one step further by designing a small molecule structure corrector to change the conformation of ApoE4 to see if it would behave more like the innocuous ApoE3 variant. After exposing the ApoE4 expressing neurons to this small molecule, they found that Alzheimer’s pathology was eliminated and the neurons were able to function normally over a longer period of time. With further research, this structure corrector could prove to be a promising therapeutic approach to treating patients with Alzheimer’s disease.






Join or login to leave a comment
JOIN LOGIN