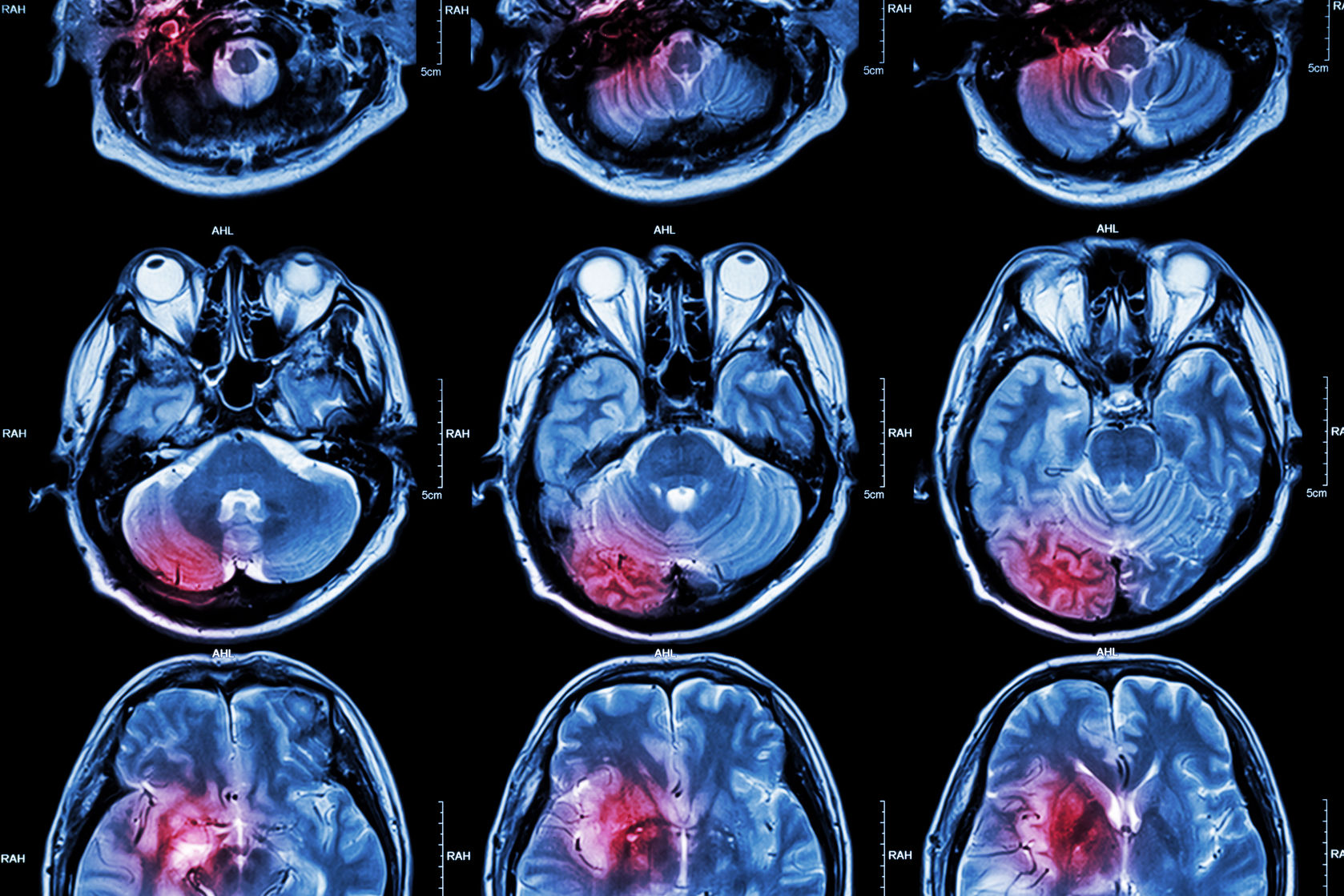
On Wednesday, Biogen announced that its experimental stroke drug natalizumab failed to improve clinical outcomes in a Phase IIb study. As a result, the biotech company will discontinue development of the drug for this indication.
“As pioneers in neuroscience, Biogen remains committed to developing treatments for people with acute neurological conditions including stroke,” said Michael Ehlers, executive vice president, Research & Development at Biogen. “While we are disappointed with the ACTION 2 study results, we have furthered our knowledge of the disease and will continue to pursue innovative approaches in this area, including BIIB093 (intravenous glibenclamide) for prevention and treatment of edema in large hemispheric infarction, one of the most severe types of stroke.”
The ACTION 2 study tested different doses of natalizumab in patients who had experienced an acute ischemic stroke. The drug failed to beat the placebo on measures of independence and activities of daily living.
RELATED: Multiple Sclerosis Drug Could be Safer for Patients if Dosing Intervals are Extended, Says Study
However, the drug was well-tolerated among study participants, which the company said bodes well for the drug’s approved indications. The antibody drug, marketed as Tysabri, is currently used to treat multiple sclerosis.
The company says that the detailed results of the Phase IIb study will be presented at a future conference.
Biogen’s stroke pipeline now consists solely of BIIB093, which is currently entering Phase III development. The drug will be tested in patients with large hemispheric infarction, a type of ischemic stroke characterized by cerebral edema, which has a high rate of mortality.
Each year, almost 800,000 individuals in the US suffer from a stroke, 87 percent of which are ischemic.






Join or login to leave a comment
JOIN LOGIN