Allergan’s blockbuster cosmetic drug Botox could soon see market competition if Mylan and Revance Therapeutics are successful in developing and commercializing their Botox biosimilar. Generic pharmaceutical company Mylan inked a deal with Silicon Valley-based biopharma Revance on the last day of February to enter into the neuromodulator market.
“This will be a significant opportunity for Mylan as we add another difficult-to-manufacture product to our pipeline,” said Mylan President Rajiv Malik. “We have reviewed the work done to date by Revance and we are extremely excited and confident about our ability to bring this important product to market.”
As part of the agreement, Revance received an upfront payment of $25 million from Mylan. The biopharma will have the potential to collect up to $100 million in milestone payments based on the successful development and commercialisation of the Botox biosimilar.
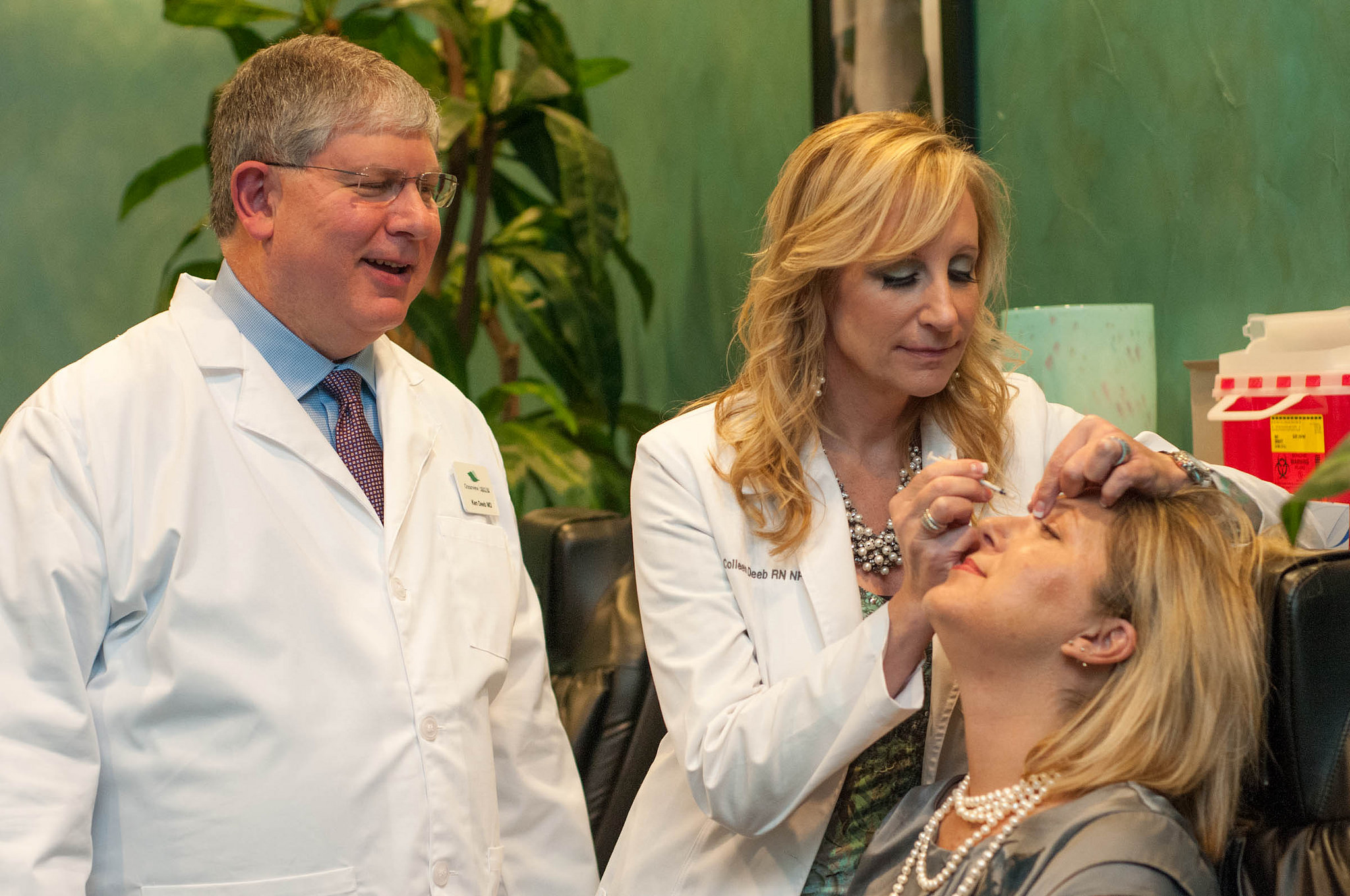
“Bringing an affordable biosimilar version of Botox to commercialization will offer patients a safe alternative to this popular and highly effective treatment,” said Malik. “Mylan is pleased to partner with Revance in the global collaboration, and share our scientific, regulatory and manufacturing capabilities and commercialization expertise.”
RELATED: Botox May See Yet Another Indication, This Time in People Who Grind Their Teeth
Mylan currently has one FDA-approved biosimilar which is a copycat version of Roche’s breast cancer drug Herceptin. Aside from Ogivri, Mylan has a biosimilar of Neulasta – a white blood cell boosting drug designed to reduce infections in cancer patients undergoing chemotherapy – in the works, which could be launched as early as late 2018.
“Global neuromodulator sales today are estimated at $4 billion and forecasted to grow steadily, exceeding $7 billion by 2024,” said Dan Browne, President and Chief Executive Officer of Revance Therapeutics. “Strategically, this partnership with Mylan allows Revance to remain focused on the development and launch of our own premium, long-acting RT002 neuromodulator, while also benefitting financially from potential future milestones and sales royalties on a short-acting biosimilar to Botox.”
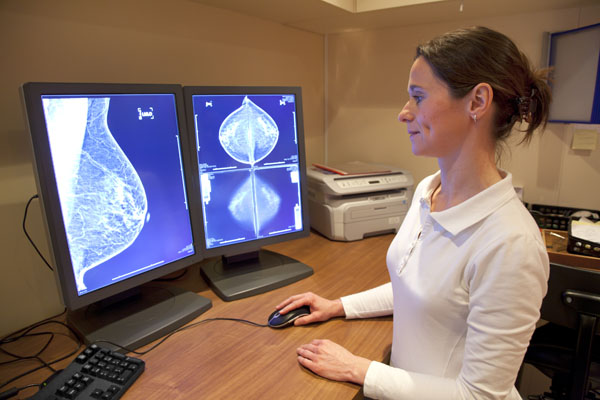
Botox is currently Allergan’s top-seller bringing in nearly $775 million in sales in the third quarter of 2017. With nine therapeutic indications, a less-expensive biosimilar version of the popular cosmetic drug could have major sales potential.







Join or login to leave a comment
JOIN LOGIN