Update (July 9, 2019): Today, Check-Cap announced the final results of their post-CE approval study of their colorectal cancer screening test. The C-Scan system could correctly identify patients with polyps ≥ 10 mm 76 percent of the time (sensitivity) compared to 29 percent of the time using a fecal immunochemical test (FIT). Across all 90 participants, the C-Scan system achieved a sensitivity of 66 percent compared to FIT’s 23 percent. Check-Cap CEO Alex Ovadia called the completion of this study “an essential milestone” as the company pursues global commercialization.
Originally published on June 25, 2019:
If you know that there’s a way to detect cancer early, would you get screened? Many people would say yes, but statistics disagree. About 40 percent of Americans do not get screened for colorectal cancer because they refuse to get or cannot get a colonoscopy. According to the American Cancer Society, 51,000 people in the US are estimated to die from colorectal cancer this year.
Faced with these dismal adherence rates, scientists have been working to develop effective and minimally invasive screening tools for colorectal cancer. One of the innovators in this space is Check-Cap, an Israeli medical device company with an aptitude for colorectal cancer prevention.
Their flagship product is an ingestible capsule that scans and sends images of the colon to an external miniaturized tracking unit worn on the patient’s back as it travels along the gastrointestinal (GI) tract. Later on, physicians can see on a viewing station if there are abnormal growths that could signify a disease.

This diagnostic device may be perceived like other capsule endoscopy products by CapsoVision, and Olympus for small bowel screening and Medtronic for colon screening, each capable of interrogating the interior GI architecture for anomalies. But, according to Check-Cap CEO Alex Ovadia, the company’s C-Scan system puts it ahead of its competition in several key aspects.
RELATED VITALS: Check-Cap Tests Colorectal Cancer-Screening Capsule in New Study
“There are lots of requirements and preparations regarding those [camera-based capsule endoscopy products] that are inconvenient,” he said in an interview with Xtalks.
The C-Scan system “does not require any bowel preparation and patients can continue with their daily routine while the capsule travels along the GI tract by natural motility.”
The C-Scan capsule possesses a “unique imaging capability” that involves low doses of X-rays which provide information about the capsule’s surroundings relative to its position. From start to finish, the patient is exposed to the same amount of radiation as a standard chest X-ray. According to Ovadia, this feature is another reason why C-Scan can be “very compelling as a screening device.”
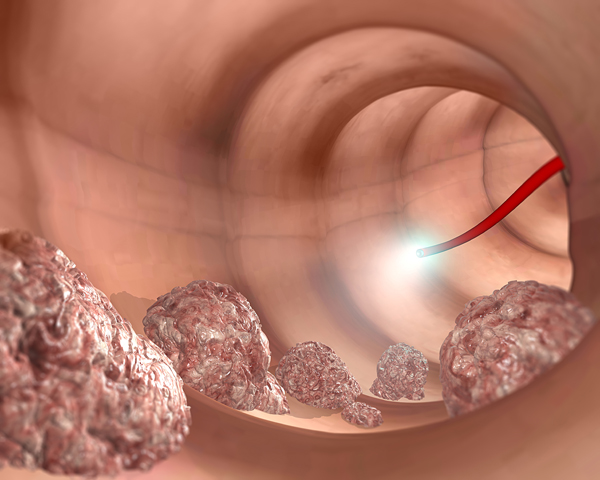
The detection of precancerous polyps is another distinguishing feature of the device. Early detection and intervention mean a better chance at survival. Other non-invasive screening tools such as stool, blood and urine tests rely on biomarkers of disease which could be too late for the patient.

“[Physicians] tell me that biomarkers are very convenient [but they’re] all aiming for cancerous polyp detection — that’s too late,” said Ovadia. “Why wait for the cancer? Why not go and perform the screening ahead of time?” Once you resect a precancerous polyp, you get a healthy individual who can live many more years, he added.
Now, the company is busy with safety and efficacy studies in sites around the world. Following CE approval last January, the company has been conducting an efficacy study in Israel, scheduled to complete this year. In May, Check-Cap researchers presented improvements to their diagnostic algorithm which now has a sensitivity of 76 percent and a specificity of 80 percent for precancerous polyps.
Currently, the device is being evaluated for patient compliance, device usability and safety in US pilot studies at New York University and, more recently, at the Mayo Clinic. These pilot studies are meant to prepare for a bigger “pivotal” study set to launch mid-2020.
Low adherence to traditional colorectal cancer screening methods is a problem all over the world. The level of non-adherence in Europe is as high as 80 percent and could be eight times lower in Asia.
“As we move ahead with our trajectory, I believe we have made some significant steps in [successfully] launching some studies in several countries including the US, to then bring this modality — this solution — to the larger population,” he said. “I believe we’re on track.”







Join or login to leave a comment
JOIN LOGIN