The FDA has approved Dexcom’s newest continuous glucose monitoring (CGM) device to help children and adults manage their diabetes. The Dexcom G6 CGM System can be used in combination with automated insulin dosing systems – also known as ‘artificial pancreas devices’ which release insulin in response to blood glucose levels – or on its own, and is the first device to receive this indication by the regulator.
“As a factory-calibrated, real-time CGM system with exceptional accuracy, the Dexcom G6 will be transformative for people with diabetes, who will no longer be required to prick their fingers for diabetes management,” said Dr. Daniel DeSalvo, Pediatric Endocrinologist at Texas Children’s Hospital in Houston, Texas. “I can tell you as someone who has type 1 Diabetes myself, with all of its features and benefits, the Dexcom G6 is the CGM device I have been anticipating for the last twenty years. This CGM system will help to alleviate the burden of diabetes management while improving the lives of people with diabetes.”
In addition to granting marketing authorization to Dexcom, the FDA has also categorized this type of CGM as a class II device, which subjects subsequent devices to a different approval pathway with less stringent requirements compared with previous diabetes devices. The ‘special controls’ established by the regulator ensure that CGM devices of moderate risk demonstrate safety and effectiveness while being approved through the streamlined 510(k) clearance pathway.
Kevin Sayer, Chief Executive Officer of Dexcom said, “The FDA’s special controls set a rigorous, new standard in our industry and clearly define the process by which other CGM systems may be approved.”
Dexcom’s CGM device was approved through the FDA’s de novo premarket approval pathway because no comparable device is currently on the market. According to the FDA, previous CPG systems were treated as high risk class III medical devices, and as such, were subject to approval through the rigorous premarket approval pathway plus general controls.
“The ability of this device to work with different types of compatible devices gives patients the flexibility to tailor their diabetes management tools to best meet personal preferences,” said Donald St. Pierre, acting director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health. “In addition, the FDA has taken steps to expedite the review process for similar, integrated CGMs and make these types of systems available to patients as quickly as possible while also helping to ensure their safety and reliability.”
Blood glucose monitoring represents a significant burden to the 10 percent of Americans who have been diagnosed with some form of diabetes. While insulin injections aren’t always required for patients with type 2 diabetes, individuals with type 1 diabetes must regularly monitor their blood glucose levels and inject themselves with insulin to make up for their body’s hormone deficiency.
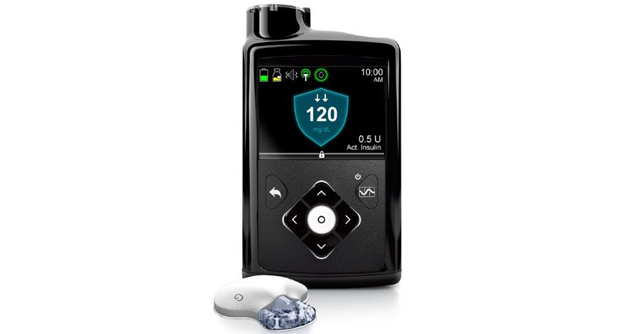
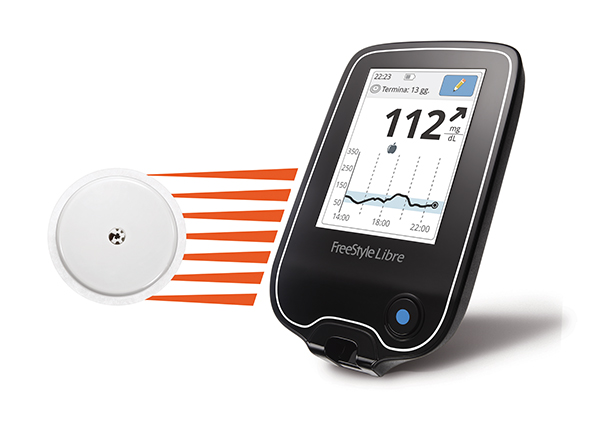
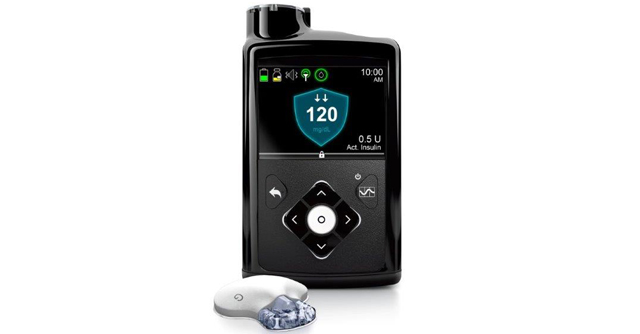
The Dexcom G6 helps to reduce the burden of fingerpricks by continuously measuring glucose levels and transmitting that data to a mobile app, alerting the patient if their blood glucose levels gets too high or drops too low. The patch attaches to the abdomen to take readings and can be integrated with an artificial pancreas system capable of releasing insulin in response to high blood glucose readings.
Unlike the first version of the device which was approved by the FDA in 2016, the Dexcom G6 CGM device is calibrated at the factory so users aren’t required to use a fingerstick to calibrate the device themselves. The diabetes device also boasts other improved features, including reducing medication interference to glucose readings by acetaminophen and 10-day wear time. According to Dexcom, their CGM device will be on the market later this year.





Join or login to leave a comment
JOIN LOGIN