Biogen made headlines in late October when they announced their intention to restart clinical studies of aducanumab after being shut down seven months prior. Since then, scientists, analysts and advocacy groups have been weighing in on whether an approval is in sight.
Phase III studies were halted in March based on a futility study predicting that the monoclonal antibody targeting amyloid beta in the brain would yield no clinical benefit to Alzheimer’s patients. However, a “new analysis of a larger dataset” showed that patients who received a high dose of aducanumab showed significant reductions in cognitive decline as measured by a validated survey. One Phase III study (EMERGE) met its primary endpoint and data from another study (ENGAGE) partially supported those findings. The company then took this revelation to the US Food and Drug Administration (FDA), who OK’d the decision to resume clinical testing.
Biogen’s tumultuous path to market has sent mixed signals to all stakeholders. On one hand, there is a great unmet need to find an effective treatment for Alzheimer’s, a progressive, neurodegenerative disorder that leads to cognitive and functional decline. A majority of treatment options deal with symptoms such as memory loss or depression, and no new Alzheimer’s drug has been approved since Forest Laboratories’ Namenda (memantine HCl) in 2003.
In an email to Xtalks, Dr. Maria C. Carrillo, chief science officer at the Alzheimer’s Association said, “On behalf of the more than 5 million Americans living with Alzheimer’s and their family members, the Alzheimer’s Association is encouraged to learn that Biogen will pursue regulatory approval from the FDA for the investigational drug aducanumab based on Phase III clinical trial results from the EMERGE and ENGAGE studies, and other related data, after finding a reduction of cognitive and functional decline in people taking the high dose.”
On the other hand, critics are skeptical of Biogen’s new analysis. Mayo Clinic neurologist Dr. David Knopman said Biogen’s application should be rejected based on data presented so far.
“The traditional way that the FDA has looked at big Phase III trials in diseases like Alzheimer’s is that you have to have two trials that are positive, and you have to meet your primary, prespecified endpoints,” Knopman told STAT News. “So, the only conclusion one can draw is that another study needs to be done. Period.”
In this case, approval could be granted to a company whose drug is supported by one, not two, trials. Does this mean the FDA is lowering its hurdles for Alzheimer’s drugs? Or should it? After all, they feel pressure from all sides to approve a therapeutic that could impact the projected 14 million Americans who could be suffering from Alzheimer’s by 2060.
“I think that any drug that is approved by the FDA for Alzheimer disease does need to demonstrate clear efficacy,” said Dr. Justin Long, clinical fellow at the Knight Alzheimer Disease Research Center in the Department of Neurology at Washington University School of Medicine, in an interview with Xtalks.
“I don’t think it has to be a curative treatment; I don’t think it has to be a drug that drastically reduces the symptoms. Any drug that can even modestly slow the course of disease progression could have a profound impact on the outcomes for patients.”
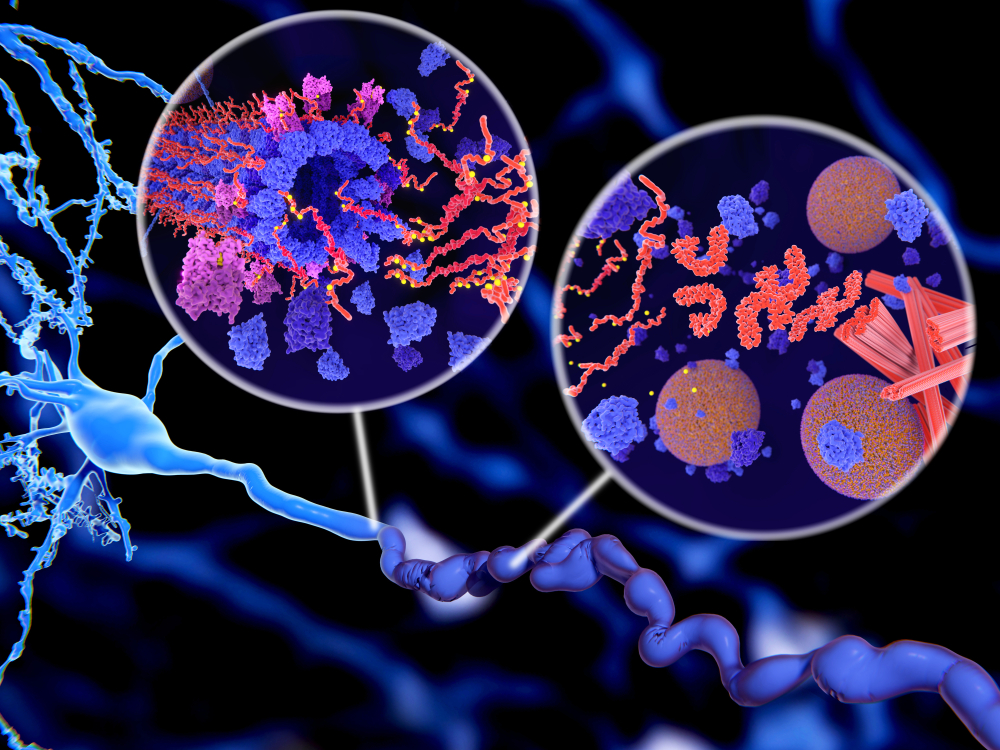
If approved, aducanumab would be the first disease-modifying drug to treat Alzheimer’s disease, plus the first to show that removing amyloid beta would lead to better outcomes. Many drug companies have attempted to target the same pathological aggregates, but so far, none have succeeded. Some companies have been axed drug programs and scaled back R&D efforts in the neuroscience space.
“The pathobiology of Alzheimer disease is extraordinarily complex,” said Long. “There are multiple different pathological species and pathways that are involved. The two major pathological proteins are amyloid-beta peptide which aggregates in the brain as well as hyperphosphorylated aggregates of the protein called tau. We’re also learning more about how neuroinflammation, the immune response of the brain to these pathological aggregates, may contribute to the disease process.”
Some emerging biotechs are picking up where Big Pharma left off. San Diego-based biotech, Samumed, develops small molecule inhibitors of key enzymes involved in multiple pathological pathways, including tau hyperphosphorylation and aggregation, and neuroinflammation. Other researchers at the cusp of clinical testing are investigating phosphodiesterase-4D inhibitors, an enzyme involved in memory formation and cognition. One of the barriers to clinical testing, however, is gathering sufficient data from preclinical models.
“Discoveries that are made in animal models that mimic Alzheimer disease have not always demonstrated success when tested in humans,” explained Long. “Being able to translate those studies is not always a simple task.”
In the last 10 years or so we have seen more preclinical studies come out demonstrating potential molecules that could move onto clinical trials, he added, referring to studies targeting the tau protein.
In terms of being first to receive regulatory approval for an Alzheimer’s drug in 17 years, Biogen might be too late. Last week, a Chinese pharmaceutical company, Shanghai Green Valley, announced they received conditional approval from the National Medical Products Administration (NMPA) for their candidate, oligomannate. The drug works by balancing the gut microbiome, which in turn reduces amyloid beta deposition and leads to improved cognitive function.
While the FDA rounds up their Peripheral and Central Nervous System Drugs Advisory Committee, Biogen plans to offer aducanumab to eligible patients previously enrolled in clinical studies. In the meantime, scientists, analysts and patients alike await the regulator’s decision with bated breath.
“Founded by caregivers, and focused on creating a world without Alzheimer’s and all dementia, the Alzheimer’s Association is an organization that puts the person with dementia and family members first,” said Carrillo. “At the same time, we believe in and are driven by scientific evidence. We eagerly anticipate reviewing a full report of findings referenced in [Biogen’s] announcement.”
Long is similarly hopeful that Biogen’s aducanumab could one-day make a difference for the Alzheimer’s patients he sees.
“If the data for aducanumab is robust, once presented, I think it’s a shot in the arm to the amyloid cascade hypothesis and targeting that specific pathway therapeutically,” said Long. “Once a disease-modifying treatment is available and approved, it’s going to dramatically change the landscape of Alzheimer’s treatment. For the first time, I will be able to offer my patients a medication to slow the rate of disease progression and cognitive decline, potentially adding years of meaningful quality of life. This is something I can’t do now.”










Join or login to leave a comment
JOIN LOGIN