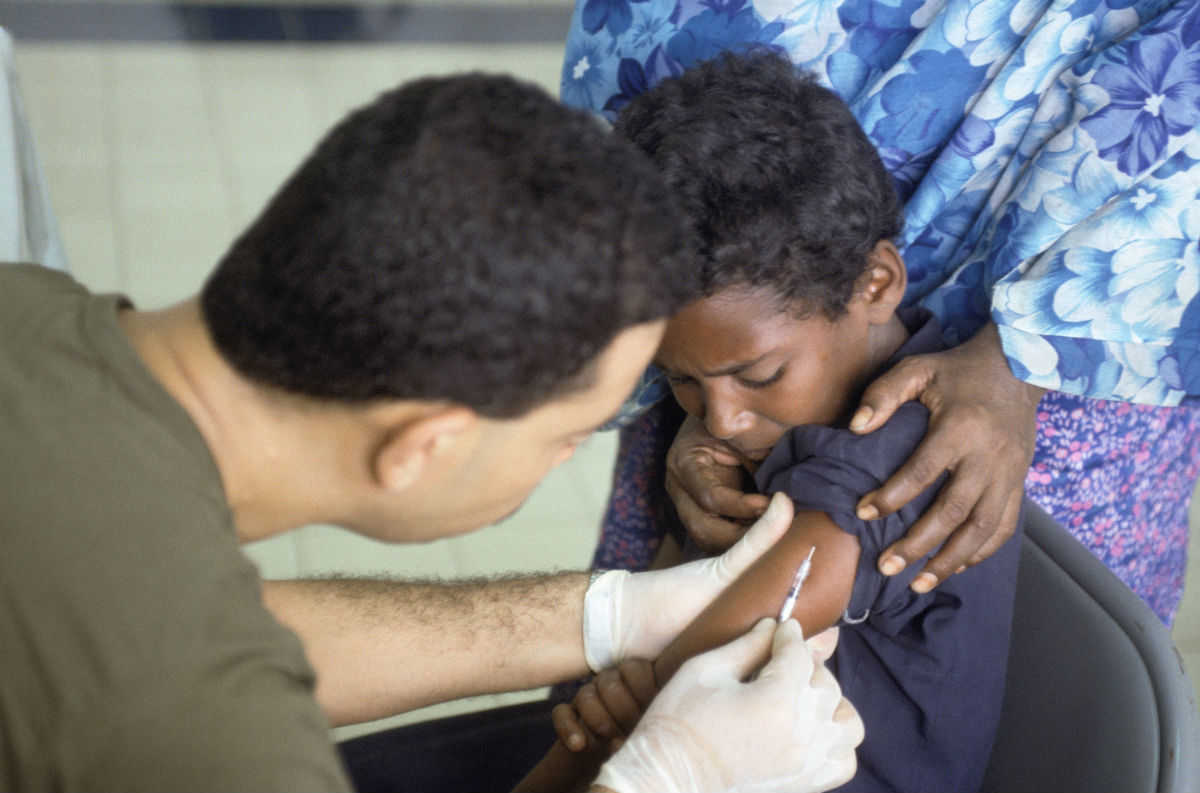
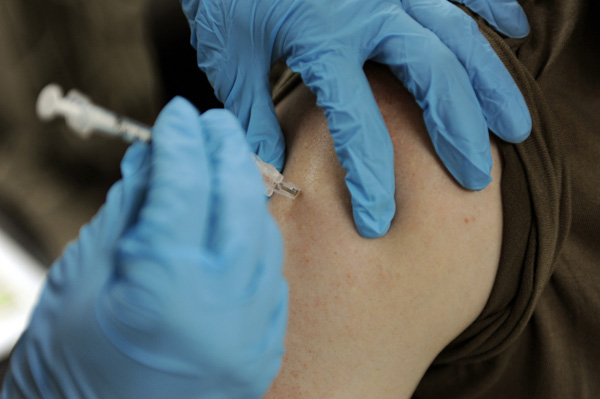
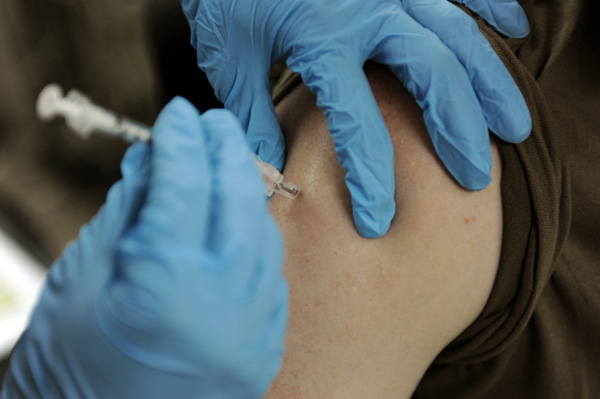
Merck’s Gardasil 9 vaccine has now been approved for patients between the age of 27 and 45 to prevent the spread of nine types of cancer-causing human papillomavirus (HPV). However, since the HPV vaccine is most effective at preventing infection when given to boys and girls before they become sexually-active, its unclear whether this approval in older adults will have a significant effect on the number of individuals who get the shot each year.
“Today’s approval represents an important opportunity to help prevent HPV-related diseases and cancers in a broader age range,” said Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research. “The Centers for Disease Control and Prevention has stated that HPV vaccination prior to becoming infected with the HPV types covered by the vaccine has the potential to prevent more than 90 percent of these cancers, or 31,200 cases every year, from ever developing.”
It’s estimated that HPV infects 14 million Americans each year, with the majority of these cases being asymptomatic, further promoting the spread of the virus. Since the link between HPV infection and certain cancers has been well-established, getting the HPV vaccine could help prevent some of the 12,000 new cases of cervical cancer each year, 4,000 of which will be fatal.
The evidence behind the HPV vaccine’s effectiveness is strong; a recent study in Denmark found that girls vaccinated with Gardasil 9 at age 15 saw a 40 percent reduction in the growth of abnormal cervical cells which could lead to cancer. Since the vaccine is now often given at a younger age – and is recommended for boys too – it has an even greater chance at preventing cancers associated with HPV infection.
RELATED: Should the HPV Vaccine Be Given to Boys Too? UK Health Officials Say Yes
Gardasil 9 is Merck’s most up-to-date version of the company’s Gardasil vaccine, which was approved by the FDA in 2006 and originally only prevented against infection with four strains of HPV. Gardasil 9 was approved in 2014 for patients between the age of nine and 26, and covers five additional HPV types.
While trials conducted by Merck have found that Gardasil is 88 percent effective at preventing HPV-related cervical cancer in women aged 27 to 45, it’s likely the company will have to do a marketing push targeting this age group in order to increase awareness. The company’s 2016 “It’s Personal: What Will You Say?” HPV awareness campaign played on parental guilt by trying to get guardians to think about how they would explain their choice not to vaccinate their child if they were eventually diagnosed with an HPV-related cancer.
According to the CDC, about half of adolescents in the US had received all recommended doses of the HPV vaccine in 2017. Depending on the patient’s age, a two-dose or three-dose schedule may be recommended.
In Q2 2018, Merck reported that global sales of Gardasil 9 totalled $608 million, up 30 percent from $469 million in the same period in 2017. However, sales in the US during this period did decline, likely due to the recommended two-dose schedule for kids aged nine to 14 years.
While the HPV vaccine in most effective when given before a patient becomes sexually active – and could be exposed to the virus – Merck maintains that those already infected with one strain could still benefit from the vaccine since it protects against eight other cancer-causing types of HPV. This could bode well for the pharma company as it looks to get adults under the age of 45 to get vaccinated with Gardasil 9.






Join or login to leave a comment
JOIN LOGIN