Janssen Pharmaceuticals Inc. has announced that it has acquired the rights to an investigational gene therapy, HMR59, from Hemera Biosciences, LLC designed to help treat a severe form of age-related macular degeneration (AMD).
The Johnson & Johnson subsidiary made the deal with Hemera — a small, privately-owned biotech company focused on developing ocular gene therapies — to help expand its eye disease portfolio and strengthen its gene therapy capabilities.
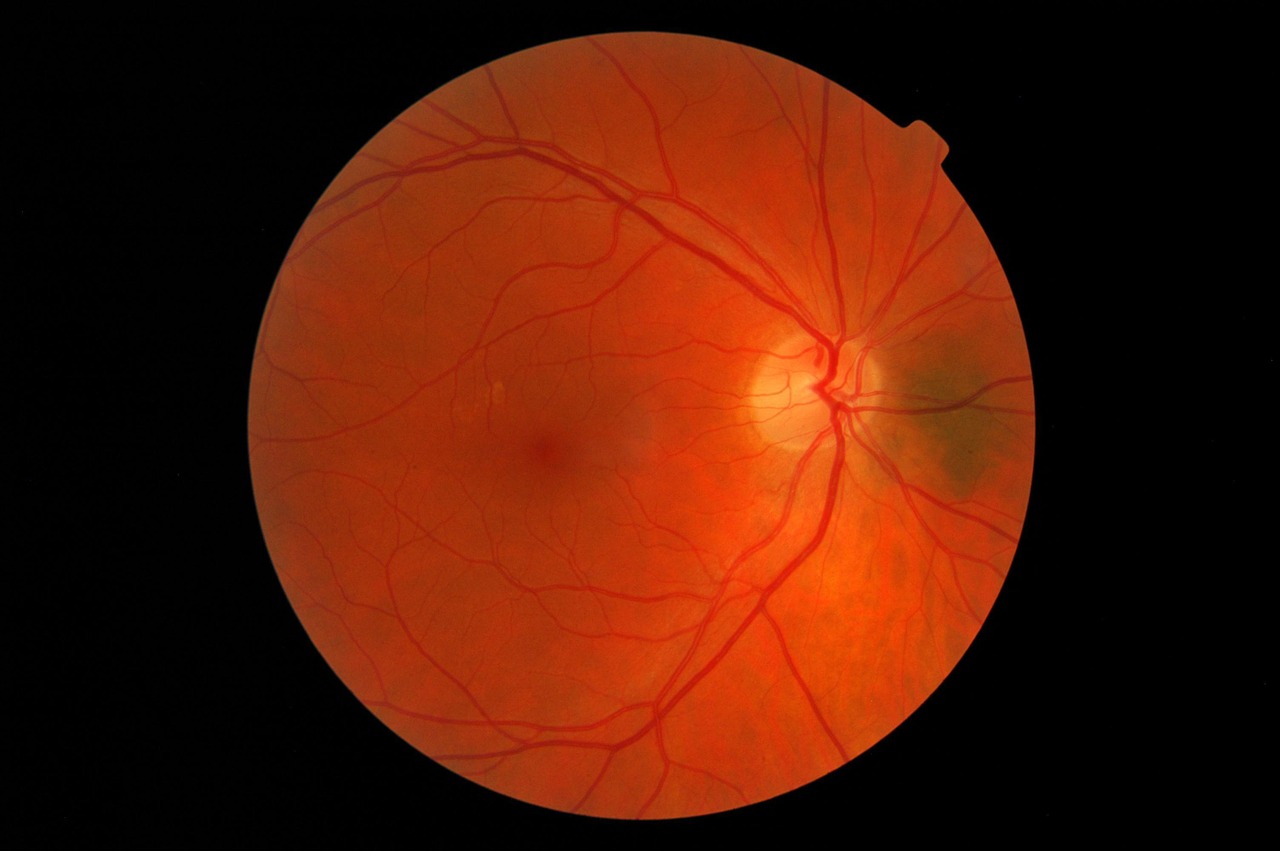
HMR59 is administered as a one-time intravitreal injection to help preserve vision in patients with geographic atrophy — a late-stage and severe form of AMD. The gene therapy was developed to treat both dry and wet macular degeneration.
Late- or end-stage AMD is the most advanced form of the disease and is the major cause of irreversible vision loss in people over the age of 60. Geographic atrophy, in particular, affects five million people globally and is a leading cause of blindness in people over 50.
Due to an aging population, the prevalence of geographic atrophy is increasing, with approximately one in 29 people over age 75 being affected by the disease, and nearly one in four over the age 90. Apart from low vision aids and vitamins, there are currently no available treatments for the condition.
Related: Study: LumiThera To Shed Light on Dry AMD
“Geographic atrophy is a devastating form of AMD that impacts the ability to accomplish everyday tasks, such as reading, driving, cooking or even seeing faces,” said Dr. James F. List, global therapeutic area head, cardiovascular & metabolism, Janssen Research & Development, LLC, in a news release from the company.
HMR59 is an intravitreal adeno-associated virus (AAV) gene therapy that is being evaluated for the treatment of dry age-related macular degeneration, or dry AMD. However, it is currently being evaluated for the treatment of both dry and wet AMD in clinical trials approved by the FDA.
“Dry AMD is a debilitating visual disease that affects millions of people with no currently available treatment options. We are very excited that Janssen recognizes the value of our HMR59 program and sees this transaction as an important moment in furthering the goal of Hemera to provide a single injection treatment for patients with dry AMD,” said Dr. Adam Rogers, chief executive officer and founder of Hemera, in a news release from the company.
Macular degeneration is a retinal disease thought to be caused by the overactivation of the inflammatory complement cascade, a key non-specific component of the immune system. The complement system consists of small proteins (named C2 through to C9) synthesized in the liver as precursors that are activated in the blood by proteases. These proteins enhance the action of antibodies and phagocytic cells to target and remove pathogens and damaged cells.
Overactivation or dysregulation of the complement system can lead to the formation of the membrane attack complex (MAC) on retinal cells to cause retinal cell death and progressive loss of vision.
HMR59 works by inducing the production of a soluble protein called CD59 (sCD59) in retinal cells that blocks the final step of the complement system. CD59 is a protective protein typically found on cell membranes that protects cells from the complement cascade. Studies have shown that patients with AMD have reduced amounts of CD59 in the retina, compromising protection against damage caused by complement.
HMR59 is injected directly into the eye to increase the ability of retina cells to make the soluble form of CD59. In this way, soluble CD59 circulates within the retina to block complement from further damaging the retina.
Janssen says that beginning with the eye, the company is rapidly developing expertise in the manufacturing, development and commercialization of gene therapies across a range of mechanisms of action, building the case for future applications to other parts of the body.
“At Janssen, we pursue the best science to uncover transformational treatments that meet patient needs, which often means pioneering the solution. This is especially the case with gene therapy and late-stage eye diseases where the paths are largely uncharted,” said Dr. Mathai Mammen, global head, Janssen Research & Development, Johnson & Johnson.
“Through this acquisition, we are blazing the trail to bring innovative solutions to patients who are losing their vision.”
While a Phase I study of HMR59 for patients with geographic atrophy is complete, a second Phase I study involving patients with wet AMD is currently conducting follow-up visits to evaluate long-term safety.
“Our aim with this novel, single-administration gene therapy is to use our development expertise and deep heritage in vision care to help improve patient outcomes by intervening early, halting the progression to blindness and preserving more years of sight,” said List.








Join or login to leave a comment
JOIN LOGIN