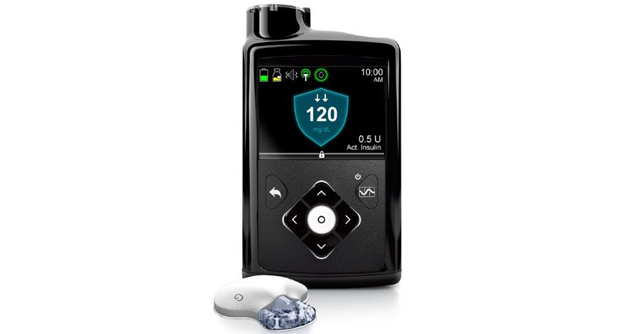
A leader in the medical device sphere, Medtronic plc announced the release of a US Food and Drug Administration (FDA) approved device called the MiniMed 770G hybrid closed loop system.
This is an insulin pump system that offers the company’s most advanced SmartGuard technology. The MiniMed 770G features technology from the MiniMed 670G but with added benefits of smartphone connectivity and an expanded age indication to include children as young as two years old.
The new hybrid closed loop therapy system benefits children with type 1 diabetes by making it easier to access and share real-time Continuous Glucose Monitoring (CGM) and pump data. Caregivers and partners are able to see the user’s data remotely on their smartphones using a proactive in-app notices system. Notices of when sugar levels are out of range are sent using the smartphone application.
Furthermore, the notices can also be automatically shared with clinicians and educators to help facilitate more effective telehealth visits and product trainings. This also helps the company, Medtronic, further enhance their software and future technology which will improve security and device features.
“We’re thrilled to be launching this new system as we understand how important these data sharing features are, particularly right now — with many individuals and families opting to see their doctors virtually via telehealth visits,” said Sean Salmon, executive vice president and president of the Diabetes Group at Medtronic, in a statement. “As a parent, I understand very personally why connectivity is so important and I’m pleased we’ll be able to broaden access to hybrid closed loop therapy with the additional peace of mind caregivers need to ensure the well-being of their loved ones. This latest launch underscores my personal commitment to making life easier for people living with diabetes through the technologies we deliver.”
The pancreas of people with type 1 diabetes cannot produce insulin. There are 1.25 million Americans with this disorder, which represents about five percent of all diagnosed cases. It is estimated that 40,000 people receive type 1 diagnosis every year in the US.
The hybrid closed loop pump is an insulin pump that is able to deliver variable basal insulin using an algorithm and a real-time CGM sensor that displays glucose trends. It helps individuals living with diabetes as it improves blood glucose levels, but input from the user is still required. This, however, is a growing space where clinical evidence is still being studied to further understand the safety of the technology and improve clinical outcomes across adults and young children.
Related: American Idol Singer, Crystal Bowersox, Helps Raise Awareness About Diabetes with Eli Lilly
In trial of the MiniMed 770G system, 151 children were assessed alongside 124 adolescents and adults over two weeks in Manual Mode and three months in SmartGuard Auto Mode, which is the hybrid closed loop algorithm. The results showed that there were no episodes of severe hypoglycemia or diabetic ketoacidosis as well as no serious device-related adverse events while using the SmartGuard Auto Mode.
“When young children are diagnosed with diabetes it is a family disease with parents and caregivers playing a substantial role in diabetes management,” Dr. Jennifer McVean, pediatric endocrinologist with University of Minnesota Health, said in a statement. “Being able to offer my patients an insulin pump system that provides safe, automated insulin delivery and smartphone connectivity is incredibly beneficial.”
The SmartGuard Auto Mode is the system that continually adjusts the amount of insulin delivered every five minutes, 24 hours a day, based on the individual’s needs. The goal is to maximize the time glucose levels are within the optimal target range and to minimize the high and low glucose levels.
Medtronic will begin taking orders for the new MiniMed 770G system in the US this week.










Join or login to leave a comment
JOIN LOGIN