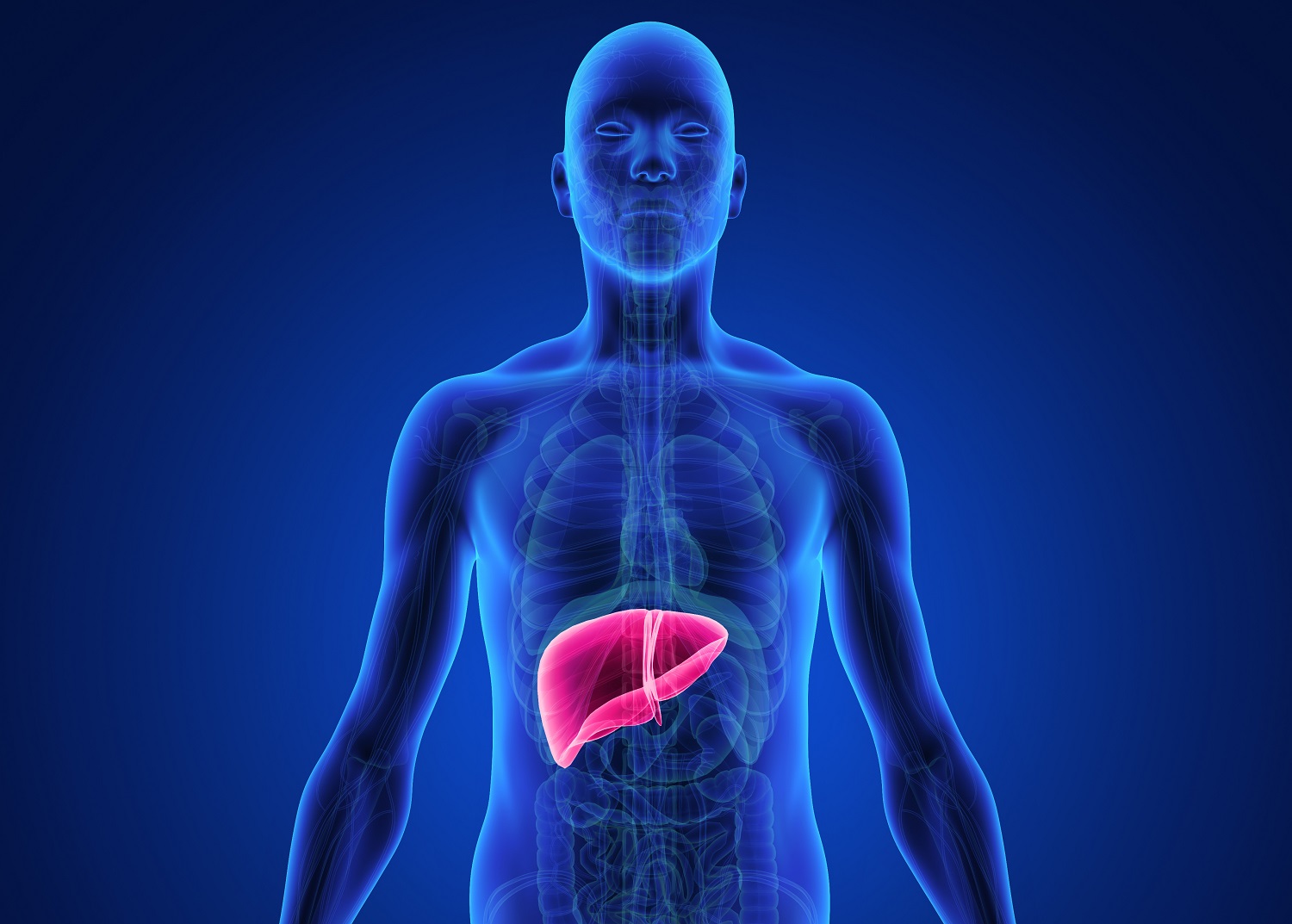
Nonalcoholic Steatohepatitis (NASH) is a chronic liver disease that affects approximately 10 percent of people living in developed countries and is considered one of the most common liver diseases worldwide. What makes the disease more deadly is that most people who are diagnosed with NASH don’t experience any symptoms. Over time, NASH could lead to cirrhosis or liver failure, at which point a liver transplant might be needed to restore function.
Despite significant investment in research and drug development, there is no cure for NASH. Since the disease has multiple features – fatty liver, inflammation and scarring – some researchers believe a combination therapy might be effective.
However, biopharmaceutical company, Durect Corporation, believes their single, small molecule agent, DUR-928, might be able to hit all the targets of NASH. At the end of March, the company announced that the first patient has been enrolled in their new phase 1b trial evaluating multiple doses of DUR-928.
“DUR-928 has been shown to have an excellent safety profile to date,” said Dr. Brent Tetri, Professor of Internal Medicine at Saint Louis University, in a press release. “As an endogenous molecule with a novel mechanism of action, it will be intriguing to see what biological signals are generated in this multi-dose study.”
In a previous single-dose study, the drug was capable of reducing the levels of known NASH biomarkers – cytokeratin-18 (CK-18), bilirubin, high-sensitivity C-reactive protein (hsCRP) and interleukin-18 (IL-18). CK-18 is considered a particularly useful serum biomarker for ascertaining drug efficacy, and is included as a primary endpoint in over 60 NASH or Nonalcoholic Fatty Liver Disease (NAFLD) trials.
The drug is being studied for its ability to reduce lipid accumulation in the liver, fibrosis or scarring and inflammation associated with NASH. As well, it could be useful in improving insulin sensitivity, which may help people with diabetes who develop NASH. This open-label phase IB study follows promising results from tests involving DUR-928 in patients with psoriasis and acute hepatitis.
“Commencing patient enrollment in this multi-dose NASH trial is the third important milestone for DUR-928 so far in 2019,” said James E. Brown, President and CEO of DURECT. “We look forward to multiple DUR-928 readouts in 2019.”
The company’s researchers intend to enroll 20 participants per treatment arm – low-dose, mid-dose, high-dose – assessing drug safety, pharmacokinetics and biological activity in people with NASH. DUR-928 is one of three investigational products in development at Durect – the fourth product is a sustained-release ophthalmology product in preclinical testing.
Last year, Pfizer and Novartis announced their collaboration on a combo therapy for NASH, using Novartis’ tropifexor and Pfizer inhibitors. Currently, Novartis has two phase II clinical trials recruiting NASH patients: TANDEM and FLIGHT-FXR.
The commencement of patient enrollment this early is a good sign for Durect. NASH trials are often crippled by low patient recruitment owing to stringent eligibility criteria and high costs. If the small molecule drug could live up to its potential, there might be new hope for those 81 million Americans affected by this disease.









Join or login to leave a comment
JOIN LOGIN