Kymriah and other CAR-T cell therapies developed by Swiss pharmaceutical company Novartis could soon be manufactured by Cell for Cure, a French contract development and manufacturing organization (CDMO). The deal is conditional upon successfully transferring Novartis’ cell therapy technology to the laboratories and potential approval of Kymriah by the EMA.
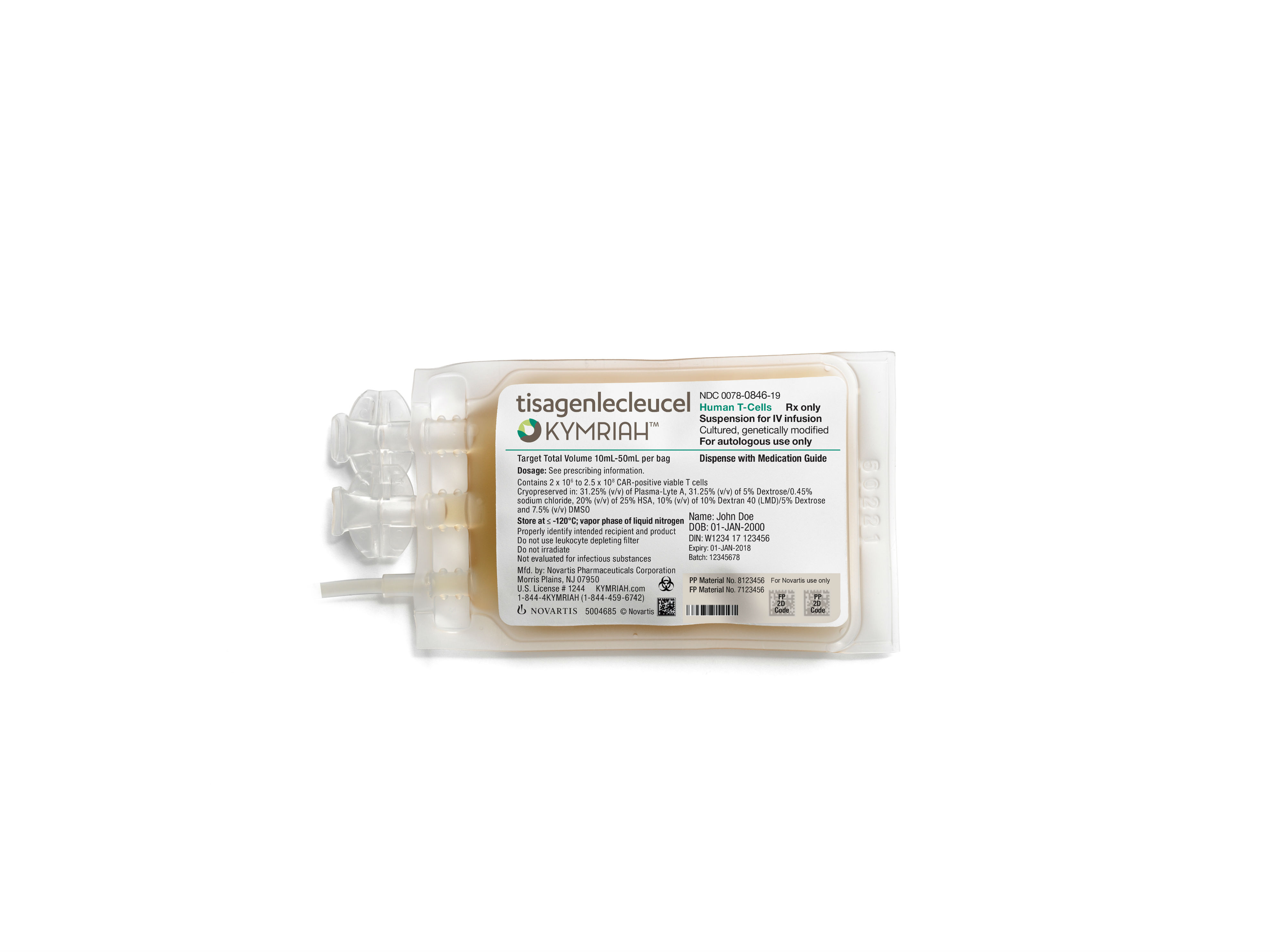
Currently, Kymriah is produced in a Novartis-owned site in Morris Plains, New Jersey, which distributes the cell therapy to patients across the US. The FDA approved Kymriah in 2017 for the treatment of acute lymphoblastic leukemia (ALL) and has since granted the drugmaker a second indication in large B-cell lymphoma.
Due to the highly-personalized nature of CAR-T cell therapies, the technology transfer from Novartis to Cell for Cure isn’t as straightforward as other contract manufacturing deals. T cells collected from each cancer patient are shipped to the manufacturing site to be engineered to recognize an antigen present on tumor cells. The altered immune cells are then shipped back to the treatment center to be reinfused into the patient. If all goes to plan, Cell for Cure could be manufacturing Kymriah in 2019 for use in both clinical trials and commercial applications.
In addition to its New Jersey plant, Novartis has enlisted the help of the Fraunhofer Institut, a site based in Germany that produces Kymriah for use in clinical trials. Securing a manufacturing hub in France would give Novartis two sites in the EU capable of creating and distributing the CAR-T cell therapy. Ideally, once this type of therapy becomes more common in cancer treatment, drugmakers should have manufacturing centers set up in strategic locations across the globe in order to serve patients in many locations and shorten the supply chain.
According to Novartis, the EMA could approve Kymriah as early as August of this year, however production at Cell for Cure wouldn’t commence until early 2019. Currently, it takes between two and three weeks to engineer and return the T cells to the patient from which they were originally harvested.
Kymriah’s bespoke nature commands a high pricetag; the single-dose therapy costs $475,000 and has already generated $12 million in sales as of the first quarter of this year.









Join or login to leave a comment
JOIN LOGIN