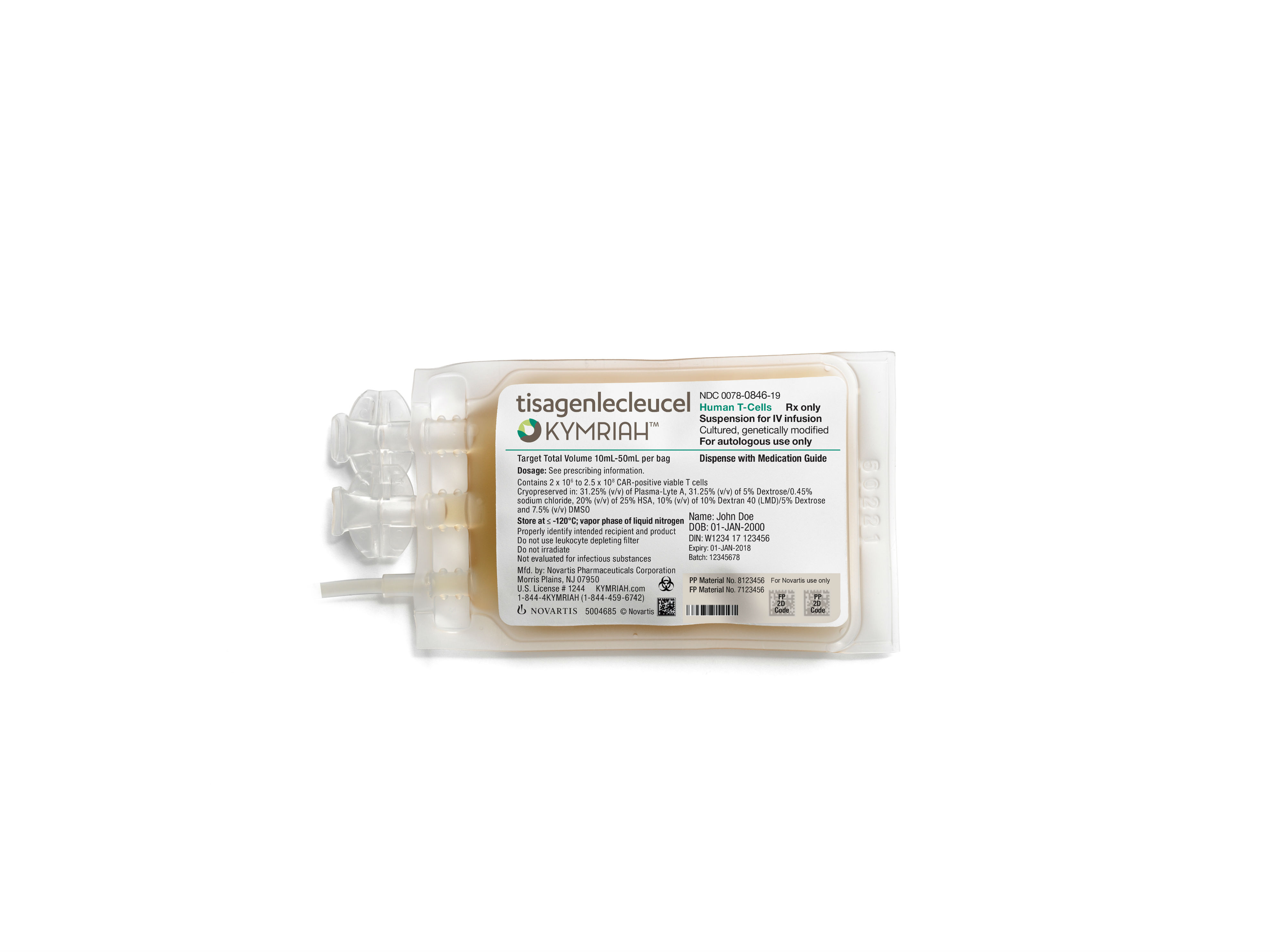
Novartis’ Kymriah has just been approved by the US Food and Drug Administration (FDA) to treat patients with large B-cell lymphoma, the most common type of non-Hodgkin lymphoma, about seven months after the CAR-T therapy received its first approval from the regulator. Originally approved in late August of 2017 for use in patients with acute lymphoblastic leukemia (ALL), Novartis’ CAR-T therapy is now the only one in the US approved for two distinct indications.
“Today’s FDA approval of Kymriah provides another opportunity for Novartis to build on its leadership in CAR-T development, delivering a potentially transformative therapy with durable and sustained response rates and a well-characterized safety profile to help patients in dire need of new treatment options,” said Liz Barrett, CEO, Novartis Oncology. “We look forward to leveraging all of our learnings and new capabilities from the initial launch of Kymriah in pediatric and young adult B-cell ALL for this larger group of patients.”
As the most common form of non-Hodgkin lymphoma, diffuse large B-cell lymphoma (DLCBL) will affect over 74,000 individuals in the US in 2018 alone, according to the National Cancer Institute. The median life expectancy for patients with relapsed or refractory forms of the cancer type, who fail to respond to first-line therapies, is just six months.
The results of the Phase II JULIET clinical trial were used to support the FDA’s decision to approve this second indication for Kymriah. The study included over 100 patients in the treatment arm, who were recruited to participate at 27 sites across 10 countries.
RELATED: First CAR-T Cell Immunotherapy Approved in US Signals Paradigm Shift in Cancer Treatment
The study investigators found that half of patients given Kymriah responded to the CAR-T therapy, with 32 percent of patients having a complete response. However, CAR-T therapies in general are not without their risks; 23 percent of patients in this trial experienced severe cytokine release syndrome, an adverse reaction that occurs when immune cells become activated and release inflammatory cytokines.
“The goal of Kymriah is to provide physicians with a therapy that has demonstrated durable response rates in relapsed or refractory DLBCL patients, a patient population that has endured multiple rounds of chemotherapy with many having experienced unsuccessful stem cell transplants,” said Dr. Stephen J. Schuster, the Robert and Margarita Louis-Dreyfus Professor in Chronic Lymphocytic Leukemia and Lymphoma Clinical Care and Research in Penn’s Perelman School of Medicine and director of the Lymphoma Program at the Abramson Cancer Center. “With this approval, physicians now have a meaningful therapeutic option that can achieve and maintain a sustained response without stem cell transplant along with a consistent safety profile.”
Originally developed by researchers at the University of Pennsylvania, Kymriah is a CAR-T therapy which uses a patient’s own T cells to create a personalized, targeted cancer therapy. Kymriah is priced at $475,000 for the single-dose therapy, and brought in $12 million in the first quarter of 2018.
Kite Pharma’s CAR-T immunotherapy Yescarta was the second drug in this class approved by the FDA in October of 2017. Just months before the approval, Kite was acquired by Gilead in a deal worth $11.9 billion.





Join or login to leave a comment
JOIN LOGIN