It’s tempting to think that replacing a patient’s failing heart with a new one will solve all of the patient’s problems. But there are many risks associated with transplant, particularly if the patient’s immune system rejects the new organ.
The consequences may be more severe for patients undergoing allogeneic bone marrow transplant, a treatment option for a number of diseases including leukemia, multiple myeloma and Non-Hodgkin’s lymphoma. Patients may be at risk of developing graft versus host disease (GVHD), in which the donated bone marrow or peripheral blood stem cells attack the tissues and organs of the transplant recipient.

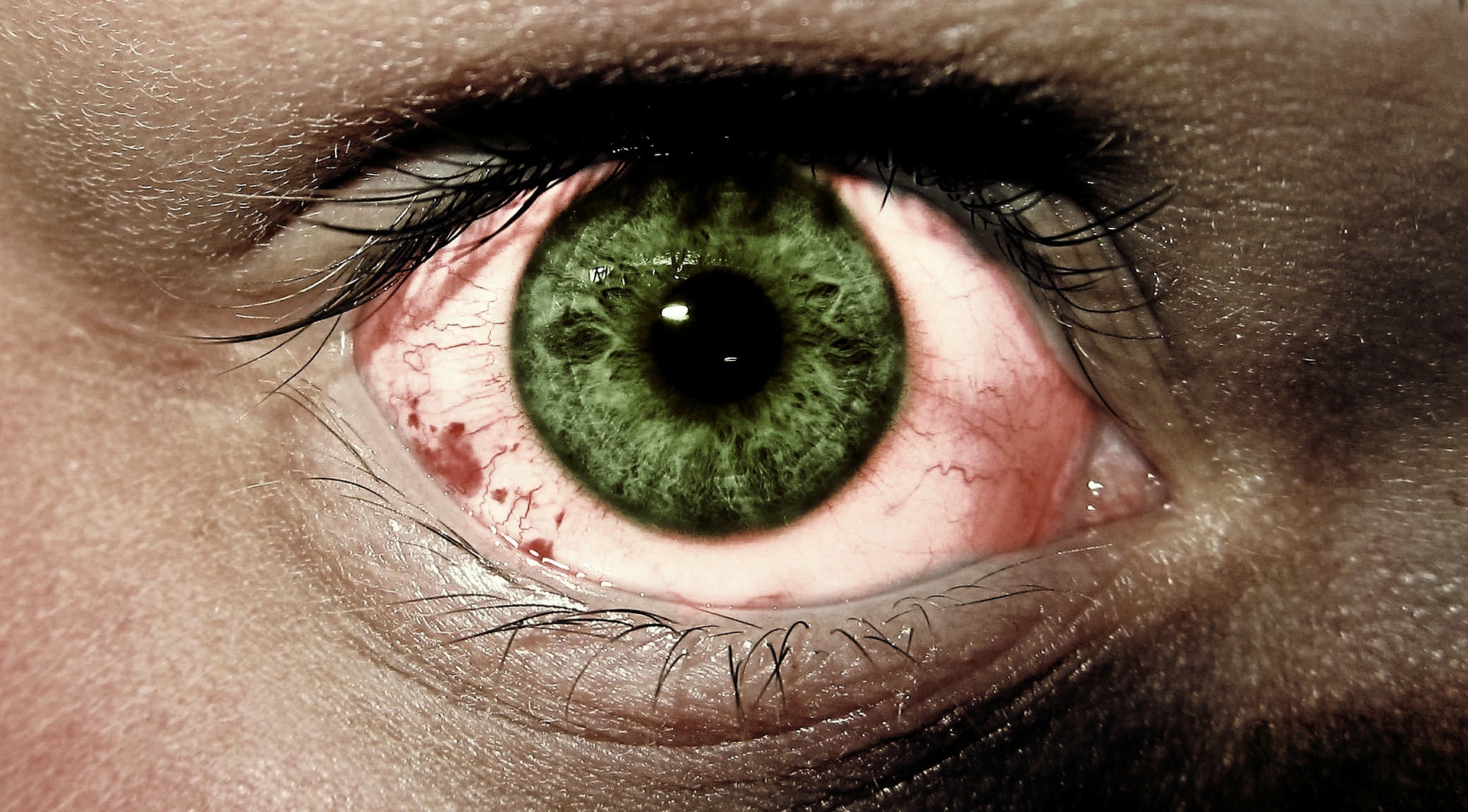
This rare but potentially life-threatening autoimmune disease can affect one or multiple organs in the body, including the eyes. Up to 60 percent of allogeneic bone marrow transplant patients develop ocular GVHD. What’s more troubling is that there is no cure or effective treatment for patients with ocular GVHD.
Dr. Shankar Musunuri, chairman and CEO of the biopharmaceutical company, Ocugen, is leading the charge to develop treatments for this underserved population.
“According to prevalence rates this year, we have around 63,000 patients suffering from this disease,” Musunuri said in an interview with Xtalks. “Even though these patients may be on oral or injectable steroids and immunosuppressants, it’s still very difficult to treat.”
Symptoms of ocular GVHD look – similar to dry eye disease, which reportedly affects up to 33 percent of the population. But the redness, inflammation and burning pain — described as “putting hot sauce on your eye and rubbing it in sand,” according to one patient — cannot be relieved with traditional eye drops.
The problem for drug developers is two-fold. First, scientists must discover or identify a drug product that can effectively treat ocular GVHD. Second, in traditional eye drop formulations, 90 percent of the active pharmaceutical ingredient is lost into systemic circulation.
Ocugen might have solved both of these problems with its flagship product, OCU300. The active pharmaceutical ingredient (API) of OCU300 is brimonidine tartrate, an agent capable of reducing inflammation, redness and eye pain. Brimonidine tartrate has long been used as a treatment for glaucoma, originally marketed as Alphagan P by Allergan. But in Ocugen’s case, OCU300 does not contain preservatives, which can be damaging to the cornea.
Another distinguishing feature of OCU300 is the way it’s delivered to the patient. OCU300 is formulated into a unique ophthalmic nanoemulsion (OcuNanoE™) to enable more active drug to reach the underlying tissue. In other words, the drug will stay on the surface of the patient’s eye longer and improves uptake to target tissues, increasing its effectiveness. Moreover, it can be formulated at a lower concentration without compromising its efficacy.

In 2017, Ocugen became the first company to receive Orphan Drug Designation from the US Food and Drug Administration (FDA) for the treatment of ocular GVHD. Now, they are halfway done enrolling ocular GVHD patients into a Phase III clinical trial evaluating OCU300 versus placebo.
“We have more than 10 centers involved,” explained Musunuri. “These are very concentrated, highly specialized clinics. While it took some time to get them up and running…we are on target to release our top-line results in the second half of this year.”
The researchers plan to follow 60 patients for 84 days, assessing ocular pain on a 10-point pain scale and ocular redness through a validated bulbar redness score which uses a digital picture.
With Orphan Drug Designation in hand and substantial safety data available for brimonidine tartrate, Musunuri anticipates a relatively swift time to market, if all goes well.
“This is a debilitating disease…people are struggling and [they] need some help,” said Musunuri. “Based on market research, payors are receptive.”
The prospect of an effective treatment option for patients with ocular GVHD is exciting, but Musunuri says it will take more than R&D to get this treatment into market.
“There are very few ophthalmologists that really understand the disease. Many people used to think, ‘this is dry eye,’ but people who deal with cancer and are specialized ophthalmologists actually understand it’s ocular GVHD. We have to do a lot of education in the field before we launch this product.”
In addition to OCU300, Musunuri and the Ocugen team are also developing gene therapies and biologics to treat retinitis pigmentosa, diabetic macular edema and wet and dry age-related macular degeneration.
“When I look into the ophthalmology space, there’s so much unmet medical need. [Even if] you look at populations with macular degeneration and diabetic macular edema, they have therapies like anti-VEGF available…but there are a significant number of non-responders to these therapies globally. So, there is a significantly underserved disease area.”








Join or login to leave a comment
JOIN LOGIN