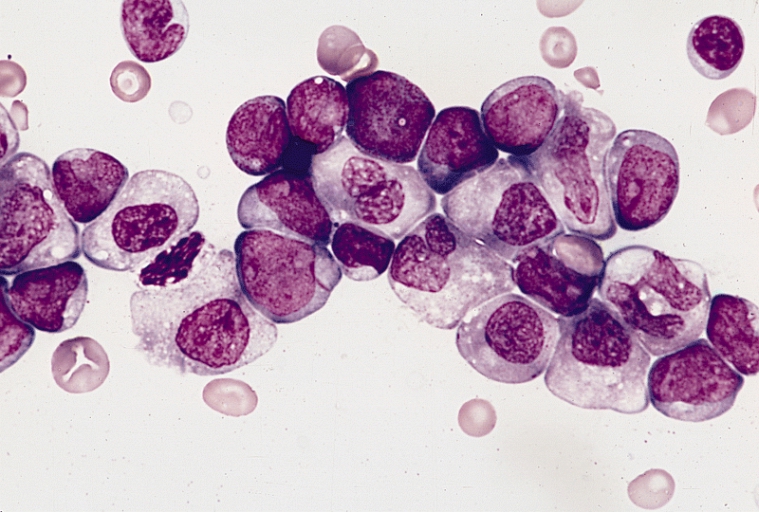
Otsuka Pharmaceutical has released results from its Phase III ASTRAL-1 study of its investigational acute myeloid leukemia (AML) drug guadecitabine (SGI-110), and they’re not what the company was hoping for. However, despite the fact that the cancer drug failed to meet the co-primary endpoints of the study, Otsuka and its subsidiary Astex Pharmaceuticals plan to continue the development program.
Patients treated with guadecitabine did not show a statistically significant improvement in complete response rate or overall survival compared to patients in the control group who were given either azacitidine, decitabine, or low-dose cytarabine, based on the treating physician’s decision. Otsuka reported that the full data will be shared during a future scientific conference, including the safety data and secondary endpoints.
The two other Phase III studies of guadecitabine – ASTRAL-2 and ASTRAL-3 – are ongoing with the aim of assessing the drug’s safety and efficacy in relapsed and refractory acute myeloid leukemia (AML), relapsed and refractory myelodysplastic syndromes (MDS) and chronic myelomonocytic leukemia (CMML).
According to Otsuka, the drug is a “next-generation DNA hypomethylating agent” which is exposed to tumor cells in a prolonged manner due to its resistance to cytidine deaminase degradation. The active metabolite in the drug is decitabine, which is capable of reversing DNA methylation in cancer cells through the action of inhibiting DNA methyl transferase (DNMT).
By removing these epigenetic modifications, the drug may reactivate tumor suppressor genes making them more susceptible to immunotherapeutic agents and other cancer drugs. The developers of this compound even believe it has the potential to re-sensitize tumor cells that have developed resistance to commonly-used chemotherapies, giving patients additional treatment options.
The ASTRAL-1 study enrolled 815 adult patients from 24 countries who were diagnosed with AML but were ineligible for intensive induction chemotherapy. Secondary endpoints – the data for which has yet to be released – included progression-free survival and duration of response, among others.
Even though guadecitabine failed to show efficacy in the current study, the company has hopes that it could prove effective against other tumor types. In addition to the ASTRAL-2 and ASTRAL-3 trials, more than twenty other studies are investigating the drug’s efficacy in both blood cancers and solid tumors, as a drug to be used on its own and as part of a combination treatment along with immunotherapy or chemotherapy.
It’s estimated that more than 20,000 new cases of AML are diagnosed each year in the US, making it the most common type of leukemia that affects adult patients. Up to 40 percent of patients under the age of 60 will fail to respond to standard intensive induction chemotherapy and will die as a result of AML. Patients over the age of 60 achieve a complete response after treatment with the standard of care less than half of the time, and Otsuka reports that it’s been over 30 years since these statistics have improved. If the results of the ASTRAL-2 and ASTRAL-3 study are more positive, adult patients with AML could one day have an additional treatment option.






Join or login to leave a comment
JOIN LOGIN