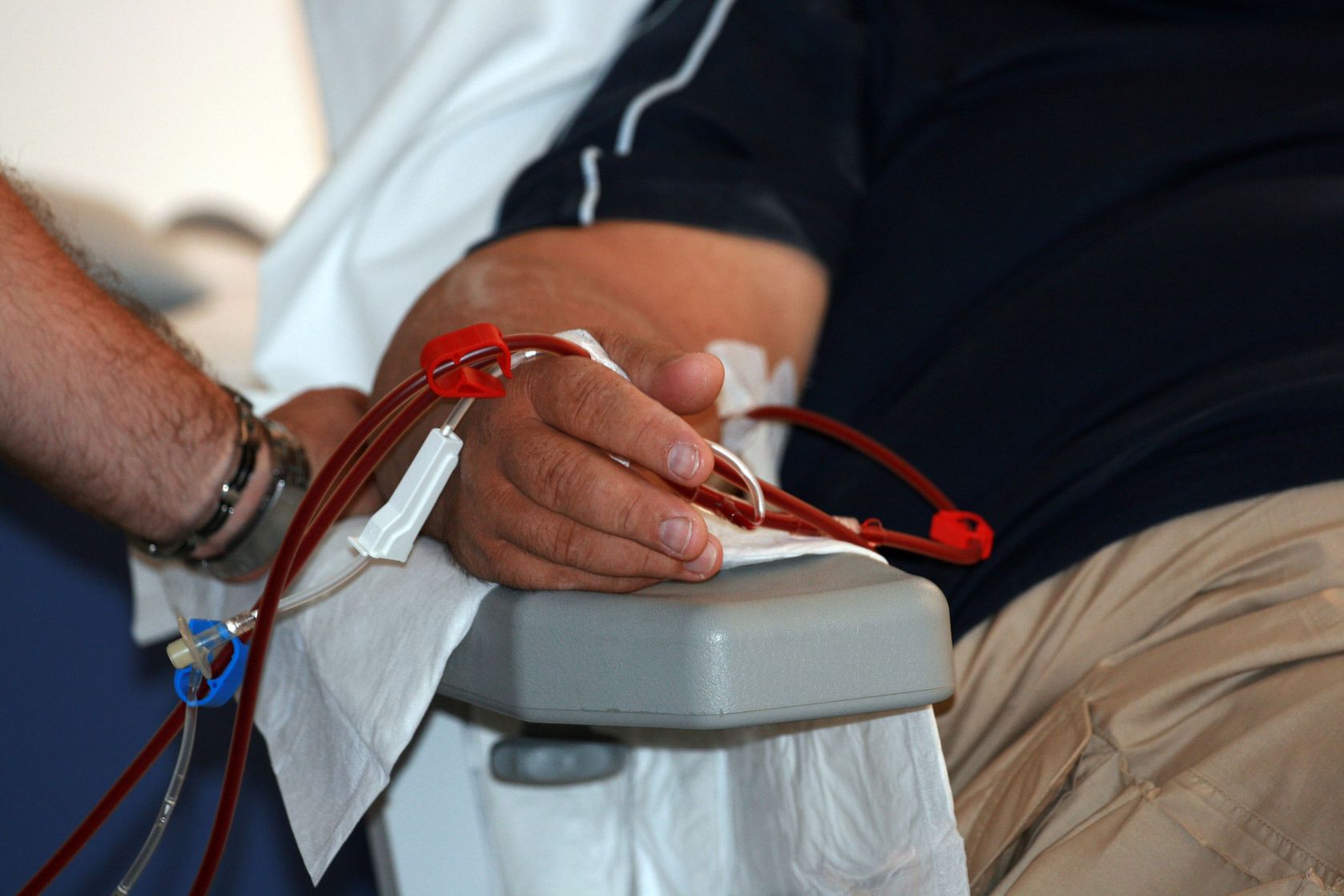
Dialysis has helped thousands of people with end-stage renal disease lead meaningful lives, but there have been few advances to improve this procedure. At best, people must spend several hours each week hooked up to a bulky machine that clears their body of wastes and toxins. The process is also resource-intensive; one patient undergoing peritoneal dialysis — where toxins are filtered through a permanent tube in the abdominal cavity — might use 5,475L (1,446 gal) of dialysis fluid each year, costing them nearly $70,000 in total.
A Singapore-based medical technology company hopes to transform dialysis with their automated wearable artificial kidney peritoneal dialysis (AWAK PD) system—a compact, lightweight device that requires only a fraction of dialysis fluid.
“The regenerative sorbent technology used in AWAK PD is an innovation with the potential to revolutionize the way peritoneal dialysis has been done in the past 40 years, providing portability and flexibility of treatment,” said principal investigator Dr. Marjorie Foo Wai Yin of Singapore General Hospital. “This technology also helps to reduce wastes and save resources by reusing dialysis fluids.”
In 2016, the team at AWAK Technologies launched a first-in-man trial, recruiting 15 people with end-stage renal disease to undergo nine sessions of AWAK peritoneal dialysis, each session lasting three to four consecutive days. Initial safety and efficacy findings were presented last Friday at the American Society of Nephrology Kidney Week in Washington, DC.
The AWAK PD system leverages so-called sorbent technology to turn used dialysis fluid into fresh fluid. An integrated infusion system reconstitutes the ‘regenerated’ fluid with the necessary solutes to maintain a continuous supply without relying on fresh volumes. According to the company, the device requires less than 2L of dialysate for an entire day of therapy compared to 8 to 12L (2.1 to 3.2 gal) with traditional peritoneal dialysis.
Not only was AWAK PD effective at removing wastes from the body, but the researchers reported no serious adverse events during or up to one month after the sessions for all 15 patients.
The findings were first reported in a news release in October 2018. AWAK Technologies’ CEO Suresha Venkataraya expressed excitement over the positive clinical evidence of this novel technology. “We are excited by the encouraging results from this important Phase I (first-in-human) trial of AWAK PD. The findings provide positive clinical evidence of AWAK PD’s safety profile as a wearable device with the potential to truly disrupt the administration of peritoneal dialysis treatments.”
At that time, the company received FDA Breakthrough Device Designation, granting them expedited development and regulatory review. With further testing, the company may champion a wearable device that delivers on-the-go dialysis to improve the lives of chronic kidney disease patients.









Join or login to leave a comment
JOIN LOGIN