In an attempt to pre-empt next year’s flu season, the National Institutes of Health (NIH) is sponsoring two Phase II clinical trials of an experimental flu vaccine in development by Sanofi Pasteur. The vaccine is designed to offer protection against the H7N9 strain of the influenza virus, which experts say has “the potential to cause a pandemic.”
The 2017/2018 flu season has been one of the worst in recent years in terms of total number of illnesses, hospitalizations and deaths. According to the Centers for Disease Control and Prevention (CDC), this year’s flu season likely peaked in early February with 7.5 percent of hospital visits across the country reported to be due to influenza-like illness (ILI).
While the CDC says the spread of influenza appears to be on the decline, they also say that many states are still reporting high numbers of cases of the flu. This flu season has been dominated by the H3N2 strain of the influenza virus which is known for its severity. To make matters worse, researchers believe that this year’s flu vaccine may be just 30 percent effective against the H3N2 strain.
“As we experience one of the worst seasonal influenza epidemics in recent years here in the United States, we also must maintain a scientific focus on novel influenza viruses, such as H7N9, that have the potential to cause a pandemic,” said National Institute of Allergy and Infectious Diseases (NIAID) Director Dr. Anthony S. Fauci. “These new clinical trials will build upon initial studies of earlier versions of an H7N9 vaccine candidate to provide a more detailed picture of its safety and ability to generate a protective immune response to current H7N9 strains.”
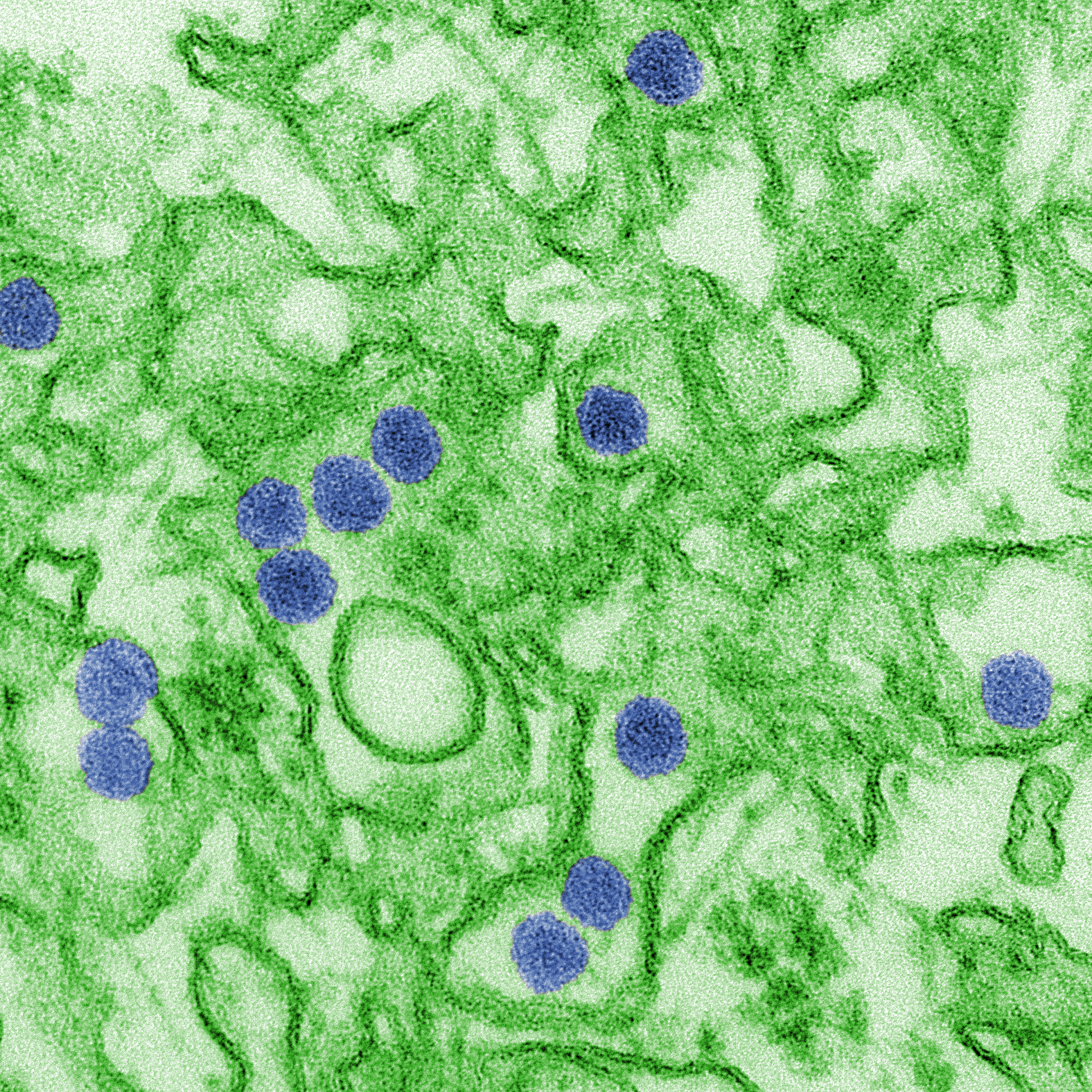
Scientists first identified the H7N9 strain of avian flu about five years ago in China. While this strain of flu has never been reported in the US, it has caused six outbreaks in China which the World Health Organization (WHO) has reported resulted in over 1,500 cases of human infection.
The H7N9 influenza virus is largely spread through contact with poultry and other birds, however mutations could allow the flu to be transmitted from person to person. Since this flu virus has a high mortality rate of nearly 40 percent, and few people have developed immunity through exposure to the virus, public health officials believe it’s imperative that a flu vaccine be developed to address this threat.
“Flu is incredibly complex and difficult to predict and this season is a somber reminder of why flu is one of the world’s greatest public health challenges,” said Dr. Anne Schuchat, acting director for the Centers for Disease Control and Prevention (CDC), in a February press briefing on flu activity in the US. “As of this week, overall hospitalizations are now the highest we’ve seen – even higher than the 2014-’15, our previous high season.”
The two clinical trials of Sanofi’s H7N9 flu vaccine will look to determine the most effective dosage of the vaccine along with the effect of an adjuvant on stimulating the immune response. Investigators are currently enrolling participants for these trials at multiple sites across the US.






Join or login to leave a comment
JOIN LOGIN