Major India-based drug company, Sun Pharmaceuticals, has debuted their dry eye disease drug to the US. The drug is a cyclosporine ophthalmic solution 0.09 percent called Cequa. This treatment offers the highest concentration of cyclosporine for ophthalmic use approved by the US Food and Drug Administration (FDA).
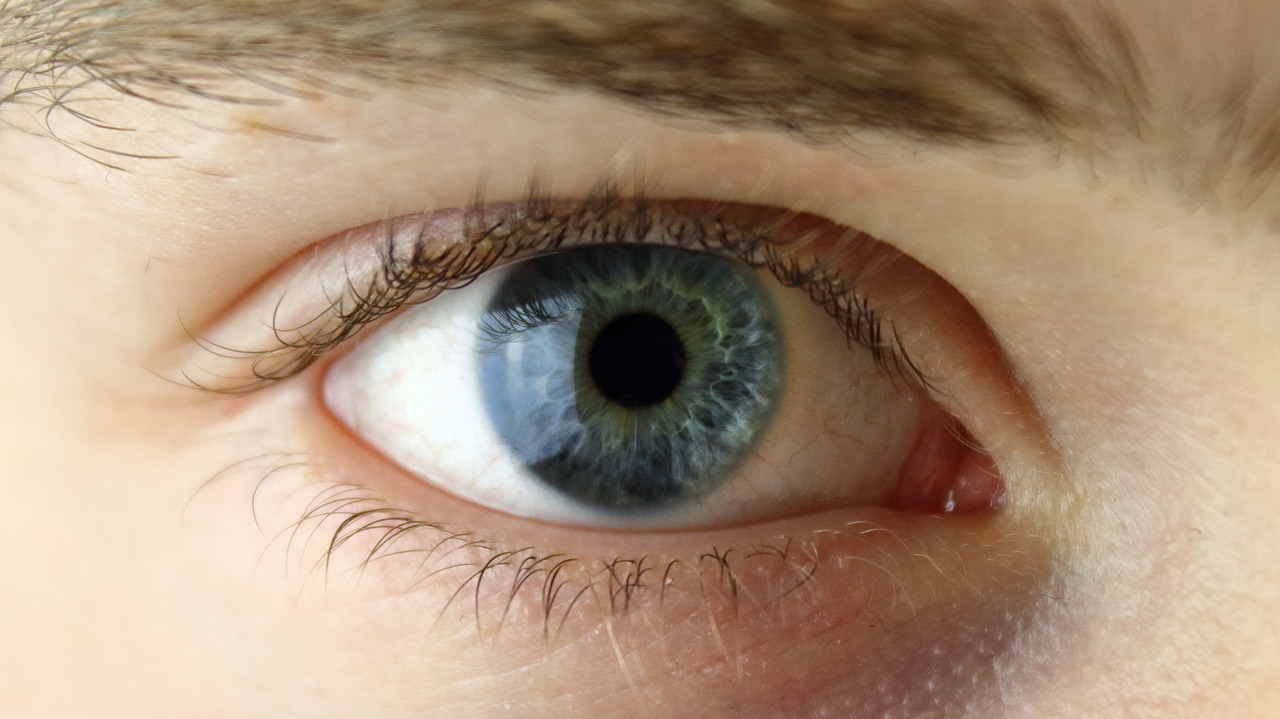
Cequa is used to increase tear production in patients with keratoconjunctivitis sicca, otherwise known as dry eye. This is an inflammatory disease that affects more than 16 million people in the US alone. Cequa is the first and only treatment to combine cyclosporine and NCELL technology to increase ocular penetration and tear penetration to ocular tissues.
According to Cequa, “NCELL uses nanomicelles composed of polymers that encapsulate cyclosporine molecules.” The small size of the nanomicelles (22 nanometers) helps facilitate the entry of the of the cyclosporine molecule for penetration into the ocular tissues.
“The US launch of Cequa, the third product in our growing ophthalmic portfolio, marks the availability of a truly innovative treatment option for patients with dry eye disease – an area with a high unmet medical need,” said Abhay Gandhi, CEO North America for Sun Pharma.
Related: Study: DNase Eye Drops Bring Relief to Severe Dry Eye Disease
Keratoconjunctivitis sicca, otherwise known as dry eye, is a condition where the tear gland does not produce enough tears to keep the conjunctiva and cornea covered by a complete layer of tears. According to the National Eye Institute, symptoms include scratchy sensations, feeling something in the eye, stinging or burning, redness, and pain. Furthermore, the risk of developing dry eye increases with age and is more common in women than men. “The availability of Cequa will enable eye care professionals to further tailor dry eye treatment to individual patients’ needs,” said founder of Tauber Eye Center in Kansas City, Dr. Joseph Tauber.
A report by Transparency Market Research states that the dry eye disease market is expected to increase at a compound annual growth rate (CAGR) of 4.5 percent between the years 2017 to 2025. The projected rise in the market is expected to increase to $7.7 billion by 2025 compared to the valuation of $5.0 billion in 2016. There is high competition in the dry eye disease market. According to Mordor Intelligence the top five competitors in the market are, Allergan PLC, Novartis AG, Santen Pharmaceutical Co. ltd, OASIS Medical and Bausch Health.
Sun Pharmaceuticals is also introducing the Cequa Support speciality pharmacy which is a program that is designed to enable commercially insured patients to easily obtain the treatment. Once a patient submits their prescription, Cequa Support will provide them with various services. These services include insurance plan benefits verification, prior authorization support, and appeals assistance. The Cequa Support program is structured to try and minimize out-of-pocket costs and provide patients with free home delivery services.





Join or login to leave a comment
JOIN LOGIN