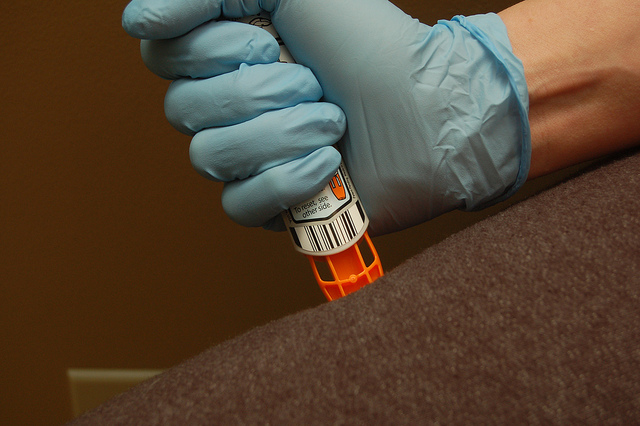
Three months following US Food and Drug Administration (FDA) approval, Sandoz and Adamis’ generic EpiPen, Symjepi, is ready to hit US markets.
The single-dose, prefilled syringe of 0.3 mg epinephrine is intended for emergency treatment of allergic reactions in people weighing 60 lbs (30 kg) or more. Its special design is also one of its selling features.
“The SYMJEPI device is small in size and fits into the palm of your hand, with the goal of a simple-to-use application and intuitive, user-friendly design,” said Carol Lynch, President of Sandoz Inc., a division of Novartis.
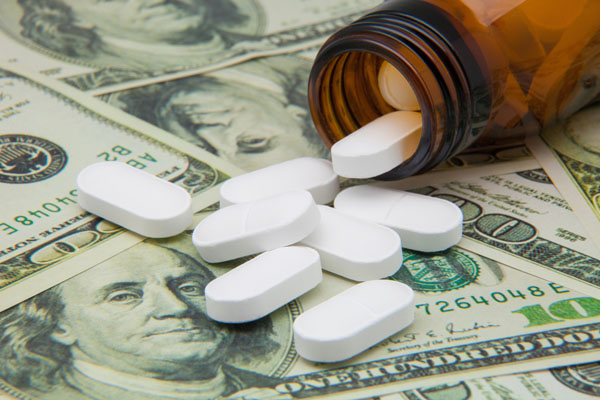
The best part about Symjepi? It boasts a (reasonably) affordable price tag of $250.00 for two syringes. Mylan’s own EpiPen Jr and Teva’s generic epinephrine injection both clock in at $300.00.
Why are epinephrine injectors so expensive? Over a decade ago, the Mylan EpiPen cost only $100, but the price tag has since increased by over 450 percent. Mylan attributed the price jump to improving product features.
It’s not to say Symjepi is the cheapest epinephrine shot on the market. At around this time two years ago, CVS began selling another EpiPen alternative called Adrenaclick for only $109.99.
According to a 2016 study, needle-and-syringe administration is a more cost-effective measure, however the force of the injection and needle depth can vary, which in turn affect the uptake of epinephrine. Alternatively, auto-injectors give more consistent injections and are thus more effective, but they tend to cost more to manufacture.
Symjepi is not an auto-injector, perhaps lending to its lower price tag.
Despite the Symjepi launch, don’t expect to find the generic EpiPen in stores just yet. Sandoz and Adamis Pharmaceuticals intend to launch the product first in an institutional setting, then in the retail market. The commercial launch of their pediatric version of Symjepi (containing half the dose of adult Symjepi) has not yet been announced, although it received FDA approval back in September 2018.
The infiltration of generics has slowly chipped away at Mylan’s monopoly on the market. According to Mylan’s 2017 year-end financial results, the EpiPen Auto-Injector decreased in sales by approximately $655.4 million. Fortunately, the company has a host of antibiotics, blood pressure medication and Parkinson’s medications to bring in profits.
The FDA has been making great strides to expedite generic drug approval and commercial launch. Just recently, the first generic version of Sabril, an anti-epileptic drug, received the nod of approval








Join or login to leave a comment
JOIN LOGIN