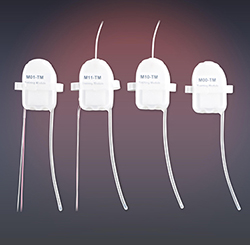
Glucose monitoring options for preclinical research have historically been limited when compared to the options available for human monitoring. As an example, Continuous Glucose Monitors (CGMs) have been prevalent in human medical applications for years; however, a continuous monitoring solution has been unavailable for preclinical applications. Existing preclinical methods are generally labor intensive and are limited by stress artifact, sample accuracy, and sampling frequency. With rodents there is also a limit to the amount of blood that can be withdrawn each day for analytical purposes.
DSI has developed a novel telemetry device for continuous monitoring of glucose, temperature and activity in rodents. The sensor is placed directly in an artery and provides direct measurements of plasma glucose. This webinar will summarize the development and beta study results associated with this novel glucose implant for preclinical research. Specific topics will include:
- General use of implantable telemetry for glucose monitoring in rats
- Results from oral glucose tolerance tests (OGTT) and intra-peritoneal glucose tolerance tests (IPGTT)
- Model development and characterization for type 1 and type 2 diabetic rats
- Dose profiles for commercially available insulin products.
A live Q&A with the audience will follow the presentation.
Key Takeaways:
- Recognize the key benefits of continuous glucose monitoring in preclinical studies
- Understand the new research applications that continuous glucose monitoring enables including
- Model development and characterization of type 1 and type 2 diabetic rats
- Oral glucose tolerance tests (OGTT) and intra-peritoneal glucose tolerance tests (IPGTT)
- Identify necessary steps to complete successful studies using telemetry as a glucose monitoring tool
Speaker

Bob Brockway, Director of Advanced Market Research, Data Science International (DSI)
Bob Brockway has 22 years of experience working in various technical and marketing roles at DSI. In previous roles at DSI he has provided strategic direction and management for DSI’s product portfolio and related marketing activities. Recently, he has enthusiastically led cross-functional efforts to commercialize a fully implantable glucose device for rodents. A key responsibility has included designing and coordinating beta testing with prominent researchers in the scientific community. Bob has undergraduate degrees in Electrical Engineering and Engineering Physics from South Dakota State University and an MBA in marketing from the University of Minnesota.
Who Should Attend?
This webinar will benefit anyone working in preclinical biomedical research who has an interest in measuring blood (plasma) glucose levels in conscious research subjects, including research scientists and technicians, veterinarians, and study directors.
Viewers will learn about the advantages of continuous glucose monitoring and the new possibilities DSI’s solution enables for diabetes and metabolic disease research.
Xtalks Partner
DSI
DSI is a pioneering biomedical research company focused on preclinical systems physiology and pharmacology. The recognized global leader in physiologic monitoring, DSI offers telemetry, instrumentation, software and services that facilitate accelerated, well-informed, drug therapy and development decisions.
DSI serves many industries including: Pharmaceuticals, Academia, Contract Research Organizations, Biological and Chemical defense, the Medical Device Industry, Government, and Biotechnology companies. We offer solutions that are tailored specifically to meet the unique research needs of our customers.
Media Partner
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account