Imaging biomarkers bring a tremendous value to clinical trials. They provide an objective means of non-invasively assessing the study participants’ physiological status. These biomarkers can be used for various purposes, ranging from inclusion criteria to measuring treatment response and monitoring adverse reactions.
Magnetic resonance imaging (MRI) is the gold standard imaging modality for examining soft tissues such as muscles, fat, and organs. In addition to providing excellent contrast between different types of soft tissue, MRI is a safe, patient-friendly, and non-invasive imaging modality free from ionizing radiation. MRI is widely available globally but sometimes perceived as challenging to implement in clinical trials, especially in multi-site settings. This webinar will challenge that myth.
In the first part of the webinar, an MRI site training specialist from Philips BioTel Research, an imaging contract research organization, will share her experience of how to successfully use MRI in clinical trials. This will highlight the clinical trial research team, best practices, and important considerations for the usage of MRI. Global multi-site settings, where site training, protocol standardization, and quality control are keys to success, will be emphasized. A firsthand look at MRI imaging in a clinical trial from the imaging facility’s point of view will be included.
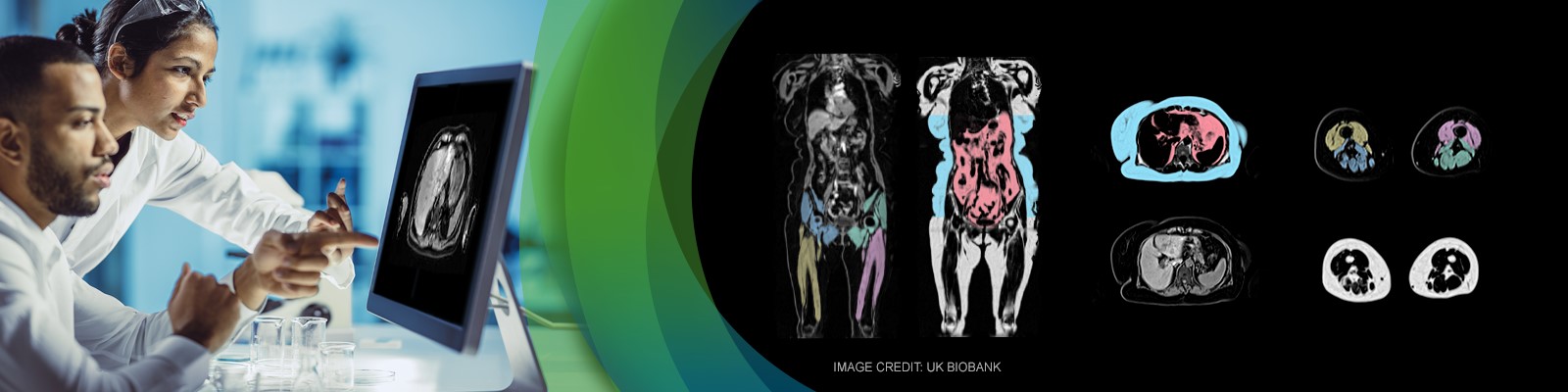
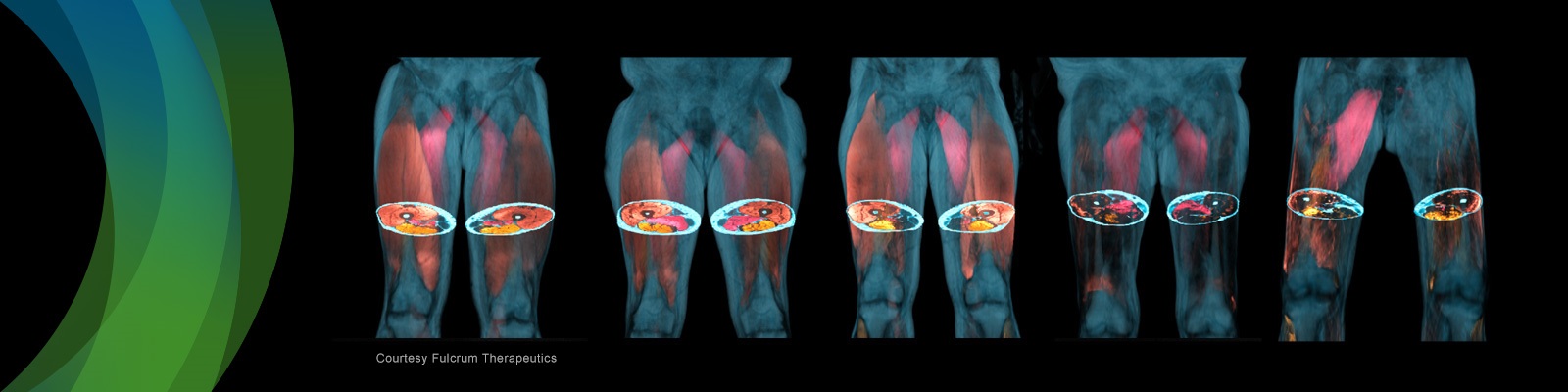
In the second part, an imaging scientist from AMRA Medical will discuss quantitative MRI biomarkers for body composition analysis. It is increasingly understood how a person’s fat distribution and muscle composition are helpful for risk assessment, diagnosis and prognosis in a multitude of diseases including muscular dystrophies, sarcopenia, obesity, liver disease, type 2 diabetes and cardiovascular disease. To successfully use quantitative imaging biomarkers in large-scale studies, high accuracy and precision have to be achieved in conjunction with extensive automation. Therefore, measures that can be taken both on the image acquisition and the image analysis side will be reviewed.
Register for this free webinar to learn about the simplicity, cost-efficiency and overall effectiveness of MRI biomarkers in clinical research.
Speakers

Elizabeth Lenio, RT (MR), (M) BS, MRI Training Specialist, Philips BioTel Research
Elizabeth Lenio is an ARRT certified MRI technologist with over 15 years of experience. She has worked at the University of Rochester and in a private office setting. She has been with Philips BioTel Research since 2014 as an MRI Training Specialist. The training team assists in image protocol development and supports imaging sites participating in clinical trials. She has vast experience conducting remote and on-site trainings globally.

John Heerfordt, PhD, Imaging Scientist, AMRA Medical
Dr. John Heerfordt is a biomedical engineer with 4+ years of MRI research experience. As an imaging scientist at AMRA Medical, he focuses on developing the company’s MRI portfolio – ranging from scan protocols to image analysis algorithms and biomarkers. He joined the team in 2021 after completing a PhD in cardiovascular MRI at Lausanne University Hospital in Switzerland. His thesis concerned novel ways of addressing cardiac and respiratory motion in MRI of the coronary arteries.
Who Should Attend?
This webinar will appeal to individuals with the following or related job titles:
- Principal Investigator
- Chief Medical Officer
- Medical Director
- Clinical Project Manager
- Imaging Specialist
- Physicians
- Clinical Operations
- Clinical Development Manager
- Procurement Manager
- Statistician
- Data Manager
- Regulatory Coordinator
- Research Coordinator
- Research Nurse
What You Will Learn
- Why magnetic resonance imaging (MRI) endpoints should be used in clinical trials
- Best practices to easily include MRI in global, multi-site studies
- Quantitative vs. Qualitative imaging biomarkers
- Phenotyping using MRI-based body composition measurements
- Image acquisition and analysis considerations for high accuracy and precision
Xtalks Partner
AMRA Medical
AMRA Medical is a digital health company at the forefront of medical imaging and precision medicine. The company has developed a new global standard in body composition analysis, delivering multiple fat and muscle biomarkers with unrivaled accuracy – all from a rapid whole-body MRI scan.
AMRA offers medical device and medical research services to support transformative care and vital decision-making, from clinical research to clinical care.
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account