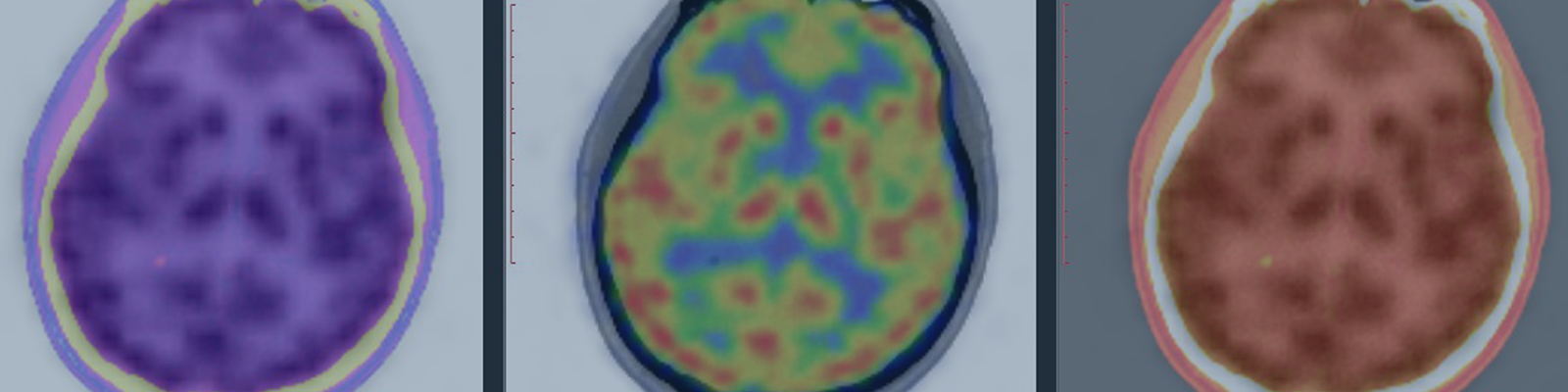
Non-alcoholic steatohepatitis (NASH) is characterized by the presence of an abnormal accumulation of fat in the liver that can progress to cell injury and inflammation. Recent intervention studies have aimed to reduce the NASH-related metabolic stress and liver fat levels by decreasing the amount of lipid droplets in the hepatocyte, before inflammation and fibrosis become irreversible. Medical imaging is a powerful tool for measuring the effect of the intervention on liver fat. To incorporate effective imaging-based experiments into clinical trials for NASH, there are several factors to consider during the experimental design phase.
In this webinar, our featured speaker will discuss the following:
- Liver biology and metabolism basics, including the progression to non-alcoholic fatty liver disease (NAFLD) and NASH
- Use of non-invasive imaging to grade and monitor fatty liver diseases
- Measurements that are key for fat fraction analysis, inflammation and elastography
- Common imaging challenges and how to account for them
- Serum markers and their uses
Speaker

Mark W. Tengowski, DVM, MS, PhD, Scientific Director, MSK & More Medical Affairs, Bioclinica
Dr. Mark Tengowski brings a large breadth of applied medical knowledge, critical thinking ability and drug development experience from compound nomination through post-marketing investigations to Bioclinica. In the industry since 1999, his imaging experience spans ultrastructural- and light-level microscopic evaluations to non-invasive imaging methods, applying imaging endpoints to nominate and advance compounds, manage safety findings and achieve regulatory approval. Mark serves as a medical advisor to the Business Development team, supporting new requests for clinical trials and consultation while being a scientific expert for projects with an inflammation or musculoskeletal focus (e.g., osteoarthritis, ankylosing spondylitis, NASH Crohn’s disease, orphan diseases and more). With a veterinary background in inflammation and pharmacology, Mark approaches protocols from a mechanistic point of view – devising an imaging strategy that will achieve the study’s objective and end points.
Mark was previously invited to speak at the US Food and Drug Administration and is the author of several draft guidance issuances. He has given posters and podium presentations at national and international meetings, participated in several disease workshops and published numerous abstracts, articles and book chapters.
Dr. Tengowski is a graduate of the University of Wisconsin-Madison and is licensed to practice veterinary medicine in WI and NY states, with USDA-accreditation, and is a member of the American College of Rheumatology.
Who Should Attend?
This webinar is suitable job titles in the following departments:
- CMO, CSO, CIO
- TA Head
- Medical Monitors
- Clinical Scientists
- Imaging or Biomarkers Scientists
- Heads of: R&D, Clinical Research, Clinical Development, Clinical Operations
- Study/Project Manager
- Protocol Manager
- Study Startup
- Procurement/Outsourcing
What You Will Learn
Join this webinar to learn about:
- Liver biology and metabolism basics, including the progression to non-alcoholic fatty liver disease (NAFLD) and NASH
- Use of non-invasive imaging to grade and monitor fatty liver diseases
- Measurements that are key for fat fraction analysis, inflammation and elastography
- Common imaging challenges and how to account for them
- Serum markers and their uses
Xtalks Partner
Bioclinica
Bioclinica is a global life sciences solution provider that utilizes science and technology to bring clarity to clinical trials – helping companies to develop new life-improving therapies more efficiently and safely. With hundreds of experienced scientific, medical, and domain experts, we bring unmatched insight across the development lifecycle of a trial. Bioclinica’s cloud-based offerings include medical imaging, cardiac safety, clinical adjudication, randomization and trial supply management, clinical trial management, electronic data capture and eSource, site and patient payments, pharmacovigilance, and a global network of investigative sites. To learn more, visit us at www.bioclinica.com.
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account