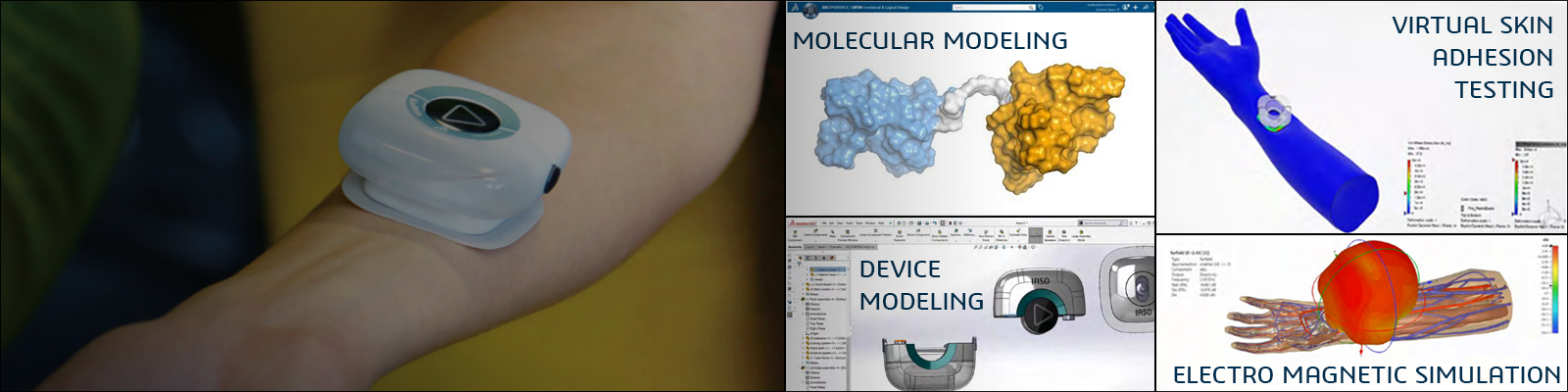
In today’s new normal, it is more important than ever for manufacturers to be resilient and sustainable in their operations. Being able to virtually validate and optimize processes, people and assets, while executing in the real world is proving to be one of the keys to successful manufacturing organization’s digital transformation.
The Virtual Twin Experience goes beyond the digital twin of a product design to include a virtual twin experience of product, processes, and delivery. Register for this webinar to discover how Life Science manufacturers can achieve this vision to adjust for new products, ramp up production, lower commissioning, and optimize their operation with quality and consistency.
Speaker

Thomas Muth, Global Strategic Industry Development Director, Dassault Systèmes
Thomas Muth is a Global Strategic Industry Development Director with DELMIA for the CPG and Life Science industries. Muth has more than 25 years of industry experience in ERP, MES / MOM and Digital Manufacturing software and technology solutions supporting operational excellence across a wide range of industries in Aerospace, Transportation, Industrial Equipment, Consumer Goods, and Life Sciences.
Who Should Attend?
- Engineers
- Designers
- R&D Technical Staff
- Clinical Researchers
What You Will Learn
- Discover key requirements for sustainable operations
- Learn from real world examples on how The Virtual Twin Experience can improve the bottom line
- See how The Virtual Twin Experience can be achieved in new and existing facilities
Xtalks Partner
Dassault Systèmes
Dassault Systèmes, the 3DEXPERIENCE Company, provides business and people with virtual universes to imagine sustainable innovations. The Dassault Systèmes 3DEXPERIENCE® platform solutions provide medical device manufacturers with the ability to effectively and efficiently manage quality issues by improving traceability and QSR/GMP/ISO compliance while eliminating non-value-added activities. This can help companies avoid compliance risk, reduce waste and deliver unmatched quality, safety and efficacy.
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account