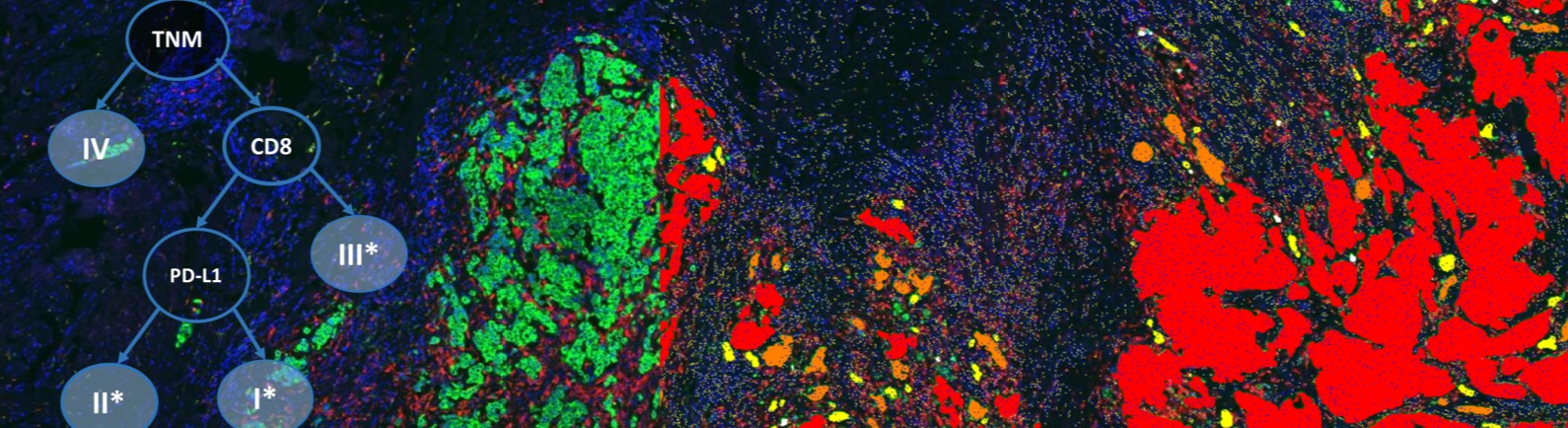
The number of clinical trials examining immunotherapy and combination therapies with immunotherapy as a backbone has exceeded 1000 in 2016. While PD-L1 by immunohistochemistry has been successfully employed as a companion diagnostic for several therapies across a handful of indications, PD-L1 is only one potential biomarker for showing a predictive response. PD-L1 does not provide a complete depiction of the tumor and it is widely documented that some PD-L1 negative patients have responded positively to immunotherapy. As more combination therapies are examined, more informative markers will need to guide when and how a treatment or combinations of treatment should be considered.
Recently, efforts have focused on the tumor microenvironment as an indicator of response to immunotherapy. Specifically, “hot” tumors with involvement of the immune system have been suggested to have a statistically higher response to treatment protocols. While some have focused on the inflammatory gene panels and mutational burden, adequately assessment of various types of T cell responders in the area of the tumor could provide insight into a patient’s prognosis. The environmental makeup remains extremely complex, with some cells enhancing immunosuppression and others inducing potent anti-tumor responses. Furthermore, evaluation of proximity and morphology of the differing types of T cells will be critical in assessing the status of the existing immune involvement.
In the case of standard immunohistochemistry, the challenge in assessment lies within the inherent semi-quantitative analysis output. Manual pathology assessment of a single IHC marker is constrained by the intensity and percent of cells stained. Additionally, standardization and reproducibility remain a challenge in the clinical trial setting. Conversely, immunohistochemistry allows for the evaluation of differential expression among the heterogeneity of the sample by which no other testing modality is suitable. Employing image analysis and machine learning from analytics software such as Definiens allows for standardization of the data captured for a given marker; thereby making the analysis of the marker under study more robust and powerful.
In the current example, a standard IHC marker is assessed and Definiens is to develop an enumeration algorithm to assess the overall intensity of the marker as well as the density of the marker in the tumor region (reported in cells/mm2). Subsequent validation shows the correlation of the analysis using the software compared with the manual assessment meets the preset criteria. Furthermore, the repeatability of the assay when using image analysis consistently meets criteria significantly better than manual assessment. Overall, the employment of image analysis for any given marker helps to improve pathology assessments and standardizes interpretation as well as systematically identifies the optimal cut-point threshold.
Speaker

Gina Wallar, PhD, MPH, Division Vice President for Pharma Services Sales, NeoGenomics, Inc.
Gina Wallar, PhD, MPH currently serves as Division Vice President for Pharma Services Sales at NeoGenomics, a leading provider of specialty testing services and solutions for clinical trials. NeoGenomics Pharma Services provides customized biomarker testing services to meet sponsors and researchers’ requirements and leverages technologies such as immunohistochemistry, florescence in situ hybridization, flow cytometry, and a wide array of molecular services. Previously, she served as the Director for LabCorp Clinical Trials (formerly Genzyme Genetics) in Los Angeles, CA for over 12 years and oversaw PD-L1 testing supporting the first companion diagnostic approval. The CLIA/CAP laboratory focused in custom testing with an emphasis on companion diagnostics solutions in oncology. Gina earned her PhD at UCLA in cancer epidemiology with her field of study on risk susceptibility polymorphisms in the cancer stem cell pathway. Her research interests include risk factors for identifying susceptibility to cancer as well as molecular prognostic indicators of disease state.
Who Should Attend?
This webinar will be ideal for Medical, Pharmaceutical, Biotech and Diagnostics executives, for directors and vice presidents of therapeutic areas and Chief Scientific, Medical and Executive Officers. It will also be informative for lab and study directors, and medical directors.
Xtalks Partner
Definiens
We improve patient lives by unlocking the tissue phenome.
In oncology, therapeutic strategies have shifted from a direct assault on cancer cells to recruiting the immune system for that purpose. Our mission is to accelerate breakthroughs for this approach by helping scientists leverage Tissue Phenomics to deepen understanding of disease biology and immune system mechanisms, to bring multi-omics data into a cancer-relevant context, and to facilitate the translation of new insights into novel therapies and treatment strategies. Our vision is to create unique patient profiles for an individualized standard of care, where patients experience fewer side effects and live longer.
Definiens’ Tissue Phenomics approach was awarded the 2013 Frost and Sullivan Company of the Year Award for Global Tissue Diagnostics and Pathology Imaging. For more information, please visit: www.definiens.com.
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account