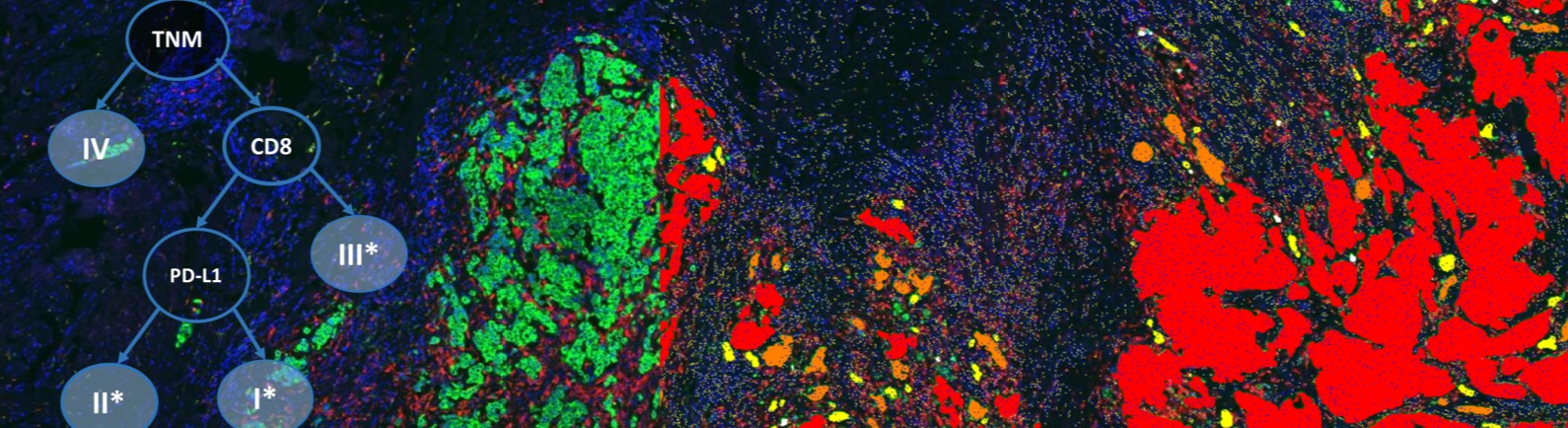
Today, IHC assay tests are commonly designed with limited stringency in comparison to common ELISA tests in the clinical setting. IHC scoring and cutpoints are determined arbitrarily biased toward the performance of the assay and then compared to patient response in hopes of finding a predictive biomarker.
In this webinar, Sharon Moulis, Principal Scientist and Director of Human Tissues Laboratory at Merrimack Pharmaceuticals, will discuss how they have devised a method of IHC assay design that increases the chance of finding the optimal cutpoint of biomarker expression and maximizing the chance of success for companion diagnostic use. Through use of standard curves and quantitative image analysis, the required target expression level can be determined and IHC assays re-optimized for more uniform and consistent scoring by a pathologist.
Speaker

Sharon Moulis, Ph.D., Principal Scientist and Director of the Human Tissues Laboratory, Merrimack Pharmaceuticals
Sharon Moulis received her Ph.D. from Yale University, studying with Dr. David L. Rimm. Her background includes development of quantitative immunohistochemistry methods, assay design, companion diagnostic strategy and implementation, and clinical trial sample management for companion diagnostics.
Who Should Attend?
This webinar will be ideal for Medical, Pharmaceutical, Biotech and Diagnostics executives from vice presidents up through Chief Scientific, Medical and Executive Officers, lab directors and medical directors.
Xtalks Partner
Definiens
Definiens is the leader in automated image analysis for life sciences and digital pathology. Founded in 1994 by Dr. Gerd Binnig, the 1986 Nobel Laureate in Physics, our mission is to enable researchers and pathologists to discover and deliver new ways to impact science and healthcare by harnessing the power of our unique Cognition Network Technology®. Definiens software solutions are used by pharmaceutical and biotech companies, universities, and cancer centers in virtually every area from discovery to preclinical development on through to clinical research and diagnostics development. We are headquartered in Munich, Germany, with a North American office in Carlsbad, California.
Media Partner
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account