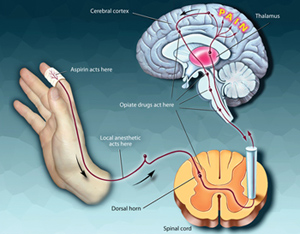
In vitro pharmacological profiling, testing compounds in select panels of assays like SafetyScreen, play an increasingly important role in reducing drug related safety attrition. In vitro screening identifies off-target activities and their adverse reaction potential by testing compounds for their activity in pharmacologically relevant targets like Serotonin 5H2B, hERG, and others and allows for early risk assessment of compounds. Failure to identify the adverse drug potential of a lead compound may have devastating consequences in later stages of clinical development.
There are several advantages of in vitro profiling; it allows optimizing compounds for specificity and selectivity enables cost effective testing of multiple compounds in parallel, uses species relevant targets and provides fast turnaround times. In vitro screening, when coupled with Pharmaco-Informatics tools can provide valuable information related to potential adverse events, therapeutic applications, repurposing and in vivo efficacy.
Speaker

Gonzalo Castillo, Ph.D., Technical Director, Eurofins Panlabs
Dr. Castillo holds an undergraduate degree in biochemistry from Universidad de Concepcion, Chile and a Doctorate degree in Molecular Pharmacology from the Albert Einstein College of Medicine. His postdoctoral work was carried out at the Dana Farber Cancer Institute, Harvard Medical School.
Who Should Attend?
Individuals with a Preclinical Pharmacology focus:
- VPs and Directors of R&D
- Lab Managers
- Principal Scientists and Scientists
From pharmaceutical or biotechnology companies and academia
Xtalks Partner
Eurofins Panlabs
Eurofins Panlabs is a global CRO specializing in discovery pharmacology testing services. We’ve been in continuous operation for over 40 years serving the Pharmaceutical and biotechnology community, setting the benchmarks for quality, convenience, and scientific expertise.
Our mission is to provide pharmacological testing services that predict clinical effects. Eurofins Panlabs’ scientific portfolio consists of over 1350 tests ranging from molecular assays, to cell-based models, through proof of concept in vivo activity determinations.
We are an extension of our client’s capabilities, providing unrivaled pharmacological expertise and knowledge, superior data reliability, and innovative solutions for drug discovery.
Media Partner
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account