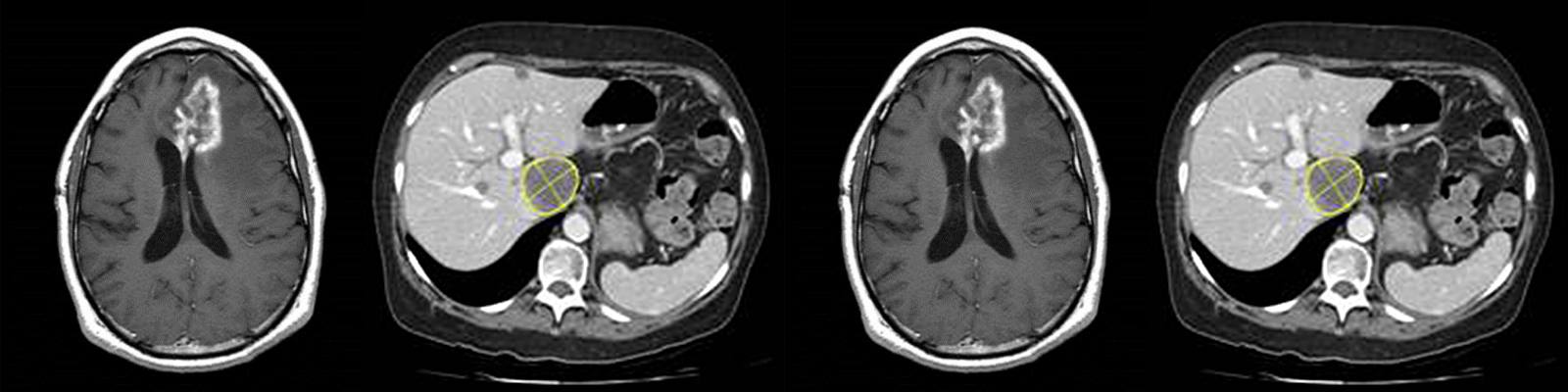
Multi-protocol programs: Are you prepared to take on the challenge of complex data acquisition parameters? Every project has a unique set of requirements that needs to be reviewed and approached carefully for successful and timely execution. For example, the study needs properly equipped clinical sites, trained technicians, experienced readers to accurately analyze the incoming data, and a proficient team ready to lead the way. A comprehensive core lab, which can efficiently execute these tasks and meet study milestones, can be a clinical lead’s greatest asset.
To a team of project managers at BioTelemetry Research, this is an exciting task of orchestrating a set of steps that leads to a cascade of events, which results in a symphony of data that brings joy to our customers and becomes part of a much greater production.
Join BioTelemetry Research’s Svetlana Kolchinsky, Director of Project Operations, for a closer look into what it takes to manage multi-protocol programs and deliver consistent results. Svetlana will be sharing operational best practices, how to successfully reduce risks, cost savings, and the benefits of partnering with a core lab.
- Next level planning for risk management and project/program oversight
- Successful project delivery through communication and early project preparation
- Key challenges and lessons learned from real life program management
Register today to discover how partnering with BioTelemetry Research ensures consistent and experienced program managers with extensive knowledge, that leads to a successful study execution.
Speaker

Svetlana Kolchinsky, Director, Project Operations, BioTelemetry Research (Cardiocore & VirtualScopics)
Ms. Kolchinsky provides operational direction to the project management, customer support and shipping and logistics functions. She leads management of the project deliverables and project execution aspects for BioTelemetry Research’s Sponsors. Ms. Kolchinsky has ten years of clinical research experience including clinical data management, site management, and laboratory management and compliance. Ms. Kolchinsky’s extensive experience in organizing and executing various aspects of the projects helps customers realize their potential and improve their clinical trial process. Ms. Kolchinsky holds a Master’s Degree in Biotechnology and an MBA in Biotechnology from Johns Hopkins University. She also possesses a Bachelor’s of Science Degree in Microbiology from the University of Maryland, College Park.
Who Should Attend?
This webinar will benefit medical and non-medical professionals in the biopharmaceutical industry, especially those supporting oncology drug development with roles in:
- Clinical Research
- Clinical Development
- Medical Affairs
- Clinical Operations
- Project Management
- Regulatory Affairs
- Procurement
- Outsourcing
Xtalks Partner
BioTelemetry Research
As a division of BioTelemetry, Inc. (NASDAQ: BEAT), BioTelemetry Research provides expert Cardiac and Imaging core lab solutions for the advancement of clinical drug development. Our cardiac network processes over 2 billion heartbeats a day, while supporting over 20,000 sites and 30,000 devices monthly, and monitoring nearly 600,000 patients and research subjects a year. We offer global operational support for cardiovascular monitoring in all therapeutic areas, and advanced imaging analyses in cardiovascular, oncology, neurology, metabolic, musculoskeletal and medical device studies. Our research team is comprised of key opinion leaders, board certified radiologists and cardiologists, sub-specialty scientists, and highly trained technicians. These experts acquire, evaluate, and report high-quality data through an efficient, cloud-based infrastructure. Additionally, we offer integrated spirometry and ECG services in cardio-respiratory clinical trials through an exclusive alliance with Vitalograph. For more information please visit www.gobio.com/research.
Media Partner
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account