See the Clinical Data Review Solution in Action:
See the Clinical Data Review Solution in Action:
-
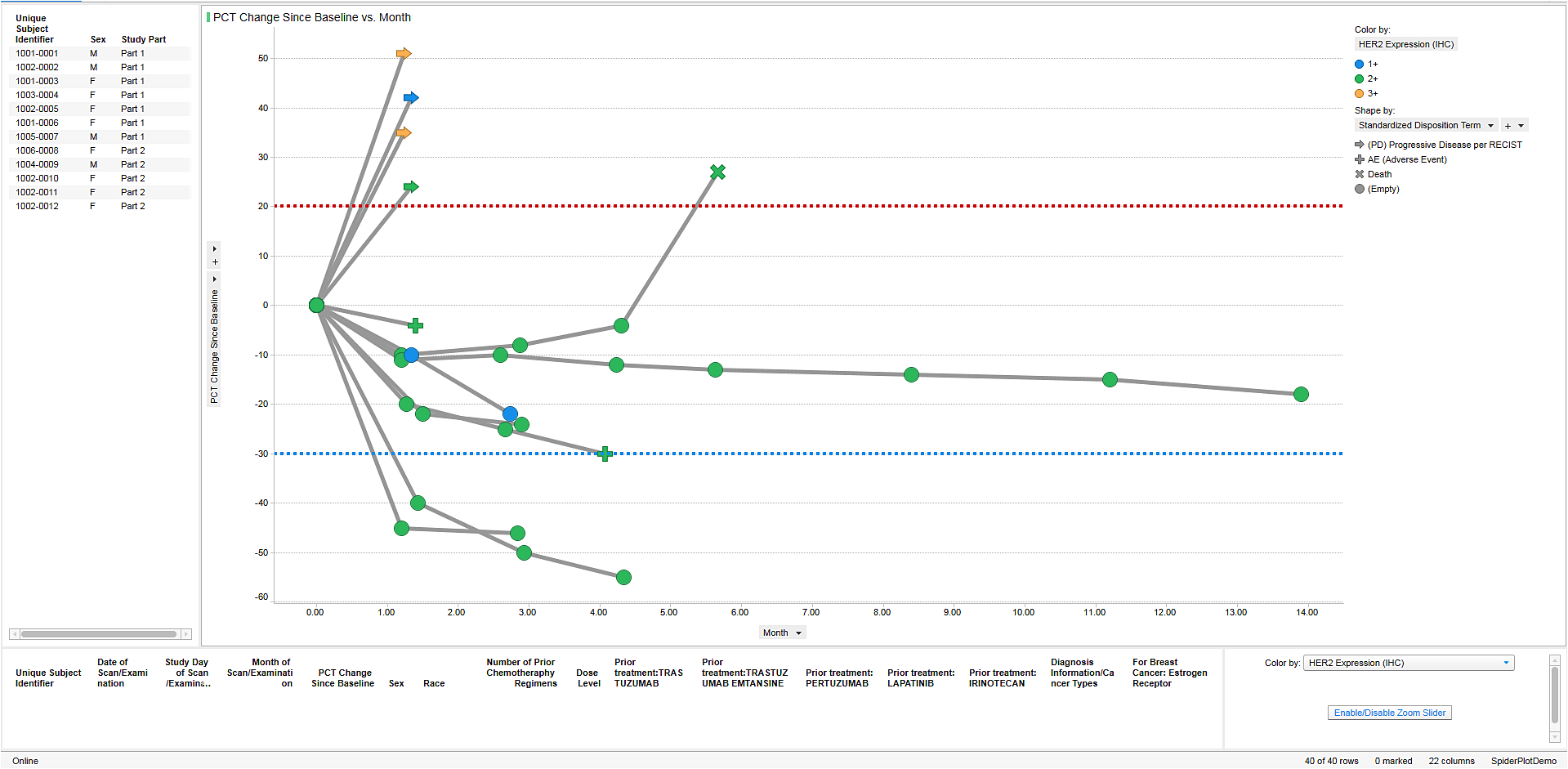
Allows merging of data across domains in an easy to use visual environment where medical monitors, safety review teams, biostatisticians, data managers, pharmacologists and others can easily identify “at risk” patients, then investigate their profiles across data domains.
-
Offers Alert Workflow: Users can set up alert criteria based on patient safety, efficacy and medical outcomes.
-
Enables Ad Hoc Workflow: Users can investigate study level data to identify patients of interest, create custom groups of patient on the fly and drill down to the cross-domain profiles.
-
Empowers team to link to individual patient narratives.
-
Includes Delta review. Line listings can be annotated as read, reviewed or amended, including user and date / time stamps.
Clinical teams are locked in a painful cycle of static report requests, slowing clinical data review and database lock. They struggle to unify and analyze data across domains; AEs, Labs, ConMeds, Demography, Exposure, etc. Incomplete clinical data access disables the ability to see “at risk” patients and make optimal decisions on safety and efficacy, early in the process.
The clinical data review solution speeds up the process, automatically combining data to allow clinical development team members to interactively explore information and discover new relationships. With the ability to quickly visualize and analyze data, team members can optimize the clinical trial process and focus their efforts on obtaining the insights and answers they need to bring drugs and devices to market faster.
Speaker

Donald Sullivan, PhD, Principal Solutions Consultant, PerkinElmer Informatics
Don joined PerkinElmer Informatics after 11 years at TIBCO Spotfire where he helped build their clinical practice and worked with both life sciences research and development customers. Prior to joining PerkinElmer Informatics, he worked in pre-sales consulting and pre-sales management positions at TIBCO™ Spotfire®, Zephyr Health, Cytobank, Ingenuity, Incyte Genomics and Proteome. Before moving over to the software world he was research scientist at Mitotix, Inc. Don earned his PhD in Cell Biology at Cornell, which was followed by a Visiting Research Fellowship at the Department of Molecular Biology at Princeton.
Who Should Attend?
Executives and clinical operation management in biopharmaceutical, medical device and diagnostics manufactures, including:
- Program, Country, Study Managers
- CRAs or Clinical Monitors
- Clinical Research Management Teams
- Clinical Trial Managers
- Sr. Manager/Director/VP R&D Clinical Informatics
- Data Managers clinical
- CROs – any level
Xtalks Partner
PerkinElmer
PerkinElmer’s advanced analytics and services solutions for Clinical Development help the world’s leading bioPharmaceutical, medical device and diagnostics manufactures discover new therapeutics faster by streamlining clinical operations, transforming risk into safety and enabling actionable decisions that can lead to better health outcomes.
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account