Multiple studies have confirmed that conducting clinical trials with a diverse range of ethnicities, genders, ages, and lifestyles is essential, since these factors can impact a treatment’s effectiveness among different groups. In the United States, legislation like the 21st Century Cares Act has recognized this fact, calling for greater clinical trial diversity.
Despite this, ethnic minorities are underrepresented in clinical trials. According to the US Food and Drug Administration (FDA), in the past 5 years, 76 percent of all clinical trial participants were Caucasians, while diverse populations such as Africans and Asians only composed 11 percent and 5 percent, respectively.
Although various solutions to this issue have been proposed, such as conducting virtual trials and hiring ethnically diverse clinical trial investigators, language ultimately plays a vital role in fostering patient diversity. This is where linguistic validation comes in.
As patients of different ethnicities and nationalities are incorporated into clinical trials, each groups’ differing linguistic and cultural backgrounds must be met in both patient outreach and documentation design, further complicating the already complex clinical trial landscape. This means that, for clinical trial sponsors, language can no longer be a secondary cost, and must be included in the drug development life cycle.
Want to learn more about how to implement the process of linguistic validation in trials? Register for this upcoming webinar to hear about the importance of this tool directly from the experts.
What is Linguistic Validation?
At its core, the goal of linguistic validation is to ensure clear patient-reported experiences are accurately communicated to study investigators regardless of cultural and linguistical differences. Linguistic validation is a multi-step process that involves translating trial materials from a source language into a target language and identifying any changes in meaning that result from translation.
Materials are then back translated into the source language and reviewed again. Once clinicians and sponsors have reviewed the translated instrument, the document enters one of the most critical steps of linguistic validation: cognitive debriefing. In a cognitive debriefing session, five to ten subjects with a disease state applicable to the clinical trial are interviewed to test their understanding of the concepts presented in a patient-reported outcomes (PRO) instrument. The end goal is for all concepts in the instrument to be equivalent across languages and cultures.
“Linguistic validation is an integral part of the push for diverse, global clinical trials,” says Joshua Maislin, Senior Customer Success Manager for the leading medical translation services company CSOFT Health Sciences. “While improved patient recruitment can help in bringing diverse patient populations in the door, linguistic validation is critical in ensuring the data provided by these patients is valid and being adequately communicated to clinical trial stakeholders.”
Here are three reasons why employing linguistic validation is key to ensuring clinical trials are diverse and the therapies being investigated will be effective for a range of different patient populations.
1. Linguistic Validation Ensures Inclusivity at All Stages of the Trial Process
The COVID-19 pandemic has shown that to produce effective treatments, patient diversity can no longer be ignored.
“Because the Black and Latinx communities make up a greater number of frontline workers, they were infected with COVID-19 at higher rates than other groups,” says Maislin. “Although these two groups were disproportionately impacted by COVID-19, they were significantly underrepresented in clinical trials for COVID treatments.”
Maislin points to the lack of participant diversity in clinical trials conducted by Gilead Sciences for its COVID-19 treatment Veklury (remdesivir) as one example of this issue. If the people who would benefit most from a treatment aren’t included in clinical research, there’s a risk that drugs won’t be effective for that patient population.
“Since different ethnicities react differently to disease and treatments, if the clinical trial data is only collected on one type of person, researchers will end up generalizing these findings to the entire population,” says Maislin. “This may lead to medicine that isn’t effective for certain groups which can have serious consequences, as pandemics and disease outbreaks last longer if only a portion of the population receives effective treatment.”
Patient participation in a clinical trial requires a leap of faith. Perhaps the drug under investigation will be more effective than therapies they’ve tried before. Maybe they’ll experience side effects. Further, for double-blind placebo-controlled trials, patients may not even know whether they’re receiving the active ingredient or not.
When it comes time to formally enroll patients in a trial, linguistic validation is useful in ensuring all participants understand the potential benefits and risks to participating in this type of research. Informed consent forms (ICFs) are another trial document that benefit from being translated so that patients understand the aims of the study, the trial protocol and what procedures will be completed during the research, ensuring that they feel comfortable voluntarily making the decision to participate.
As trial designs have started to become more patient-centric, PROs have become increasingly important in assessing participants’ experience in a trial. These self-reported measures typically assess changes in symptoms and functioning as well as quality of life as seen from the patient’s perspective.
Tools used to assess PROs are more likely to collect accurate data from patients when the language used has been linguistically validated. It’s essential that these instruments assess measures that are relevant to the target patient population, and that translated versions of PRO instruments are clear and understandable to the individuals responding to them.
2. Linguistic Validation is More Accurate Than Other Forms of Translation
Trial materials may need to be translated from one language to another because the study is being conducted in multiple countries with different national languages, or the recruitment of participants for whom English is not their native language is prioritized in order to foster trial diversity. Either way, it’s not enough to simply generate a verbatim translation without context.
Though artificial intelligence (AI) is remarkably accurate when it comes to generating machine translations for over 100 languages, currently available tools are missing the nuance needed to accurately translate these technical documents.
For example, using Google Translate to translate one of the questions (Figure 1) included on the Kidney Disease Quality of Life Instrument (KDQOL) from English to Armenian (Figure 2) produces confusing results. When the Armenian text is translated back to English, the meaning of the sentence is unclear as some colloquialisms don’t directly translate between languages (Figure 3).

Figure 1: The ninth question on the Kidney Disease Quality of Life Instrument (KDQOL).
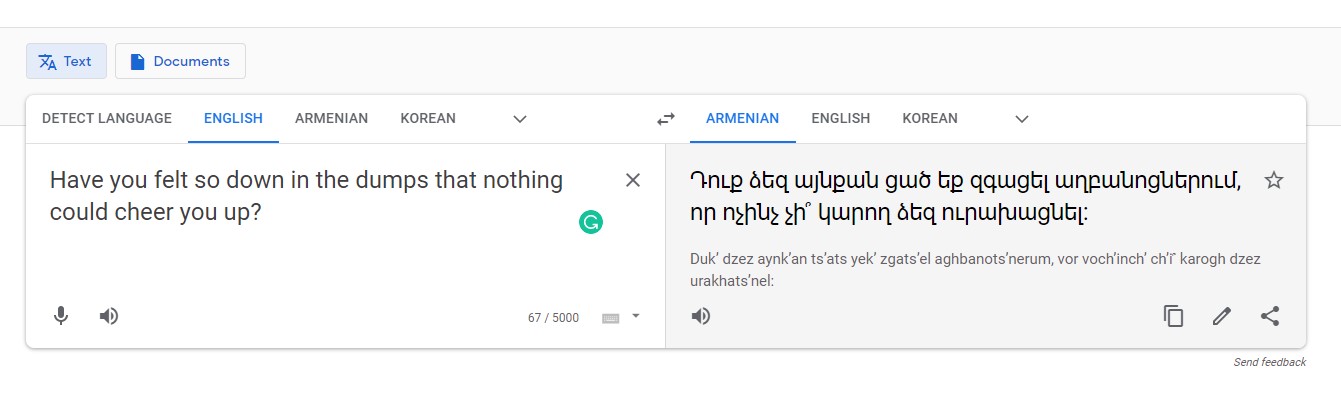
Figure 2: Google Translate results for question excerpt from KDQOL from English to Armenian.
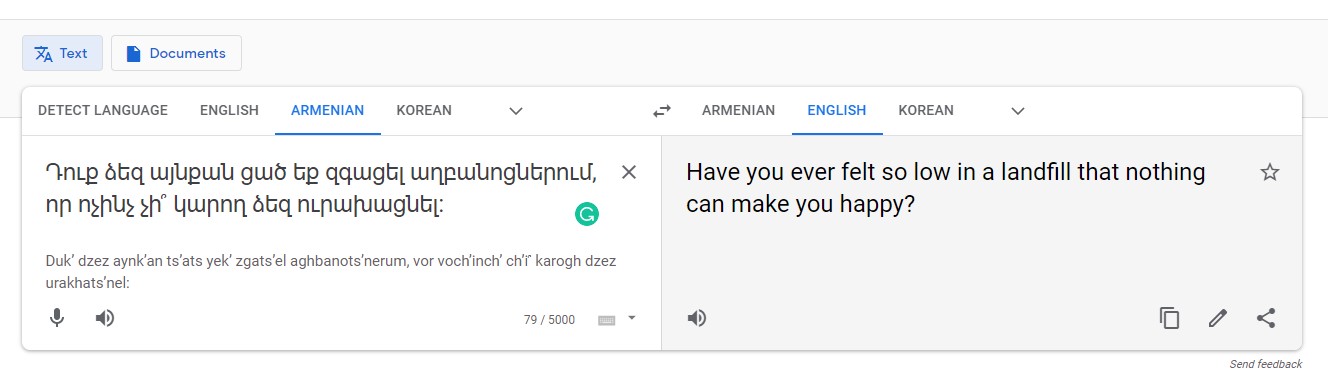
Figure 3: Google Translate results from reverse transcription from Armenian to English show colloquialisms don’t always stand up to cross-cultural interpretation.
While some recent studies have suggested that this tool may be accurate enough to translate trial results, it’s unlikely to be sufficient to translate study materials provided to patients to help them with informed consent, and reporting their own experiences via PROs.
Experienced, in-country linguists and subject matter experts are needed to ensure documentation is understandable to patients whose native language is different. This eliminates ambiguity and guarantees that the meaning of the text is consistent across different languages.
Linguistic validation is a critical tool in the effort to ensure patient diversity and develop a robust understanding of the patient experience across global populations. By adapting PRO instruments to a variety of linguistic and cultural demographics, drug development companies gain a broader and more in-depth understanding of the patient experience at the clinical trial stage.
3. Linguistic Validation Safeguards the Validity of Trial Data
Data equivalency is the name of the game when it comes to conducting global trials in countries where language and culture vary among participants. If PROs data collected from patients speaking different languages cannot be accurately compared and combined, sponsors run the risk of having to throw out one dataset altogether, losing valuable information which could be used to support regulatory applications.
Incorporating PROs into clinical trials is an increasingly popular way to engage patients participating in the clinical research process. However, if these instruments are only translated based on content, without taking local culture into consideration, study investigators may be taking one step forward and two steps back in terms of patient-centricity.
Imagine being a Mandarin-speaking patient who is asked to complete a questionnaire that assesses changes in functionality through daily living tasks. If a question asks about their ability to use a knife and fork, but this individual’s cultural norm is to use other utensils, like chopsticks, the question can be unintentionally alienating.
The rigorous process of linguistic validation allows for this question to be translated in terms of both language and culture to ensure that it’s understandable — and applicable — to all patients involved in the study.
“By ensuring cultural equivalency across all patient reported experiences, this data can be accurately compiled and analyzed regardless of the locale and language in which it was collected,” says Maislin.
This insight into patient symptoms and quality of life can also inform label claims on the approved product, as well as provide valuable information on how to adapt to specific geographical markets.
What’s more, this process integrates seamlessly with the current decentralized clinical trial landscape, which is expected to persist even after the end of the pandemic. According to Maislin, while in-person interviews for cognitive debriefing are preferable, moving this to a virtual environment makes the process of recruiting interviewees and conducting the interviews faster and more efficient. This matters because even though many trials have gone virtual, timeframes are still tight when it comes to making sure studies stay on track.
Case Study: Inclusion of Asian American Patients in Diabetes Trials
There are numerous examples of how some diseases affect those of different ethnicities more than others. A striking example of this is diabetes, which disproportionately affects Asians compared to other ethnicities. China and India currently top the list of countries with the greatest number of individuals with diabetes, and Pakistan is rapidly overtaking the US for the number three spot.
What’s more, the disease presents itself differently in Asians, with experts like Dr. George L. King, founder of the Asian American Diabetes Initiative and Senior Vice President of the Joslin Diabetes Center, asserting that diabetes actually presents at a lower Body Mass Index (BMI) in this group compared to Caucasians. For this reason, he recommends Asian Americans get screened if they reach a BMI of 23 — two points lower than traditional guidelines for what is considered “overweight.”
Despite this, ethnic and racial minorities have largely been excluded from trials in the US, leading drugmakers to develop new insulins and other diabetic drugs that may be ineffective in patients of Asian descent. Perceived language barriers likely play a large role in the lack of Asian representation in diabetes trials, which is a challenge that could be overcome by a thorough, culturally sensitive translation process.
In recent years, prominent drugmakers have taken note of this disparity and have started to take steps toward designing more inclusive trials. Diabetes drugmaker Novo Nordisk has recently conducted focused trials in patients of Chinese and Indian heritage. Another example is Eli Lilly, a company with a solid history in patient-centricity, which has run diabetes studies aimed at increasing the enrollment of Asian patients.
How to Support Patient Diversity Now
So why isn’t linguistic validation already part of every clinical project manager’s toolkit? According to Maislin, the time it takes — which runs anywhere from a few weeks to over a month — is often underestimated, so it’s important to plan for sufficient lead time to ensure the process is completed before study sites are active.
But if the industry is to put buzzwords like “patient-centricity” and “patient experience” into practice, linguistic validation will play a key role in making that a reality. What’s more, this process is actually recommended by the FDA to adapt PROs to other languages and improve patient diversity in trials.
“Linguistic validation is one of many powerful tools for clinical trial stakeholders to make good on the concepts of ‘patient-centricity’ and ‘patient experience,’” says Maislin. “In addition to ensuring valid data collection, linguistic validation also serves to adapt important clinical trial tools to study participants’ cultural, linguistic, and educational backgrounds. Without this step, the experiences of diverse patient populations may not be adequately communicated and analyzed.”
To learn more about how linguistic validation can support patient diversity in clinical trials from Maislin and his colleagues, register for this free upcoming webinar.
For those interested in learning more on how to support patient diversity in advanced clinical trials, the world-renowned Joslin Diabetes Center will be hosting their virtual event A Taste of Ginger on May 16 at 7pm EST. All proceeds, including donations and ticket sales, will go to furthering efforts in inclusive diabetes care and research.
This content was created in collaboration with the sponsoring company and the Xtalks editorial team.








Join or login to leave a comment
JOIN LOGIN