Allergan received US Food and Drug Administration (FDA) approval for a new delivery method for its popular dermal filler, Juvéderm VOLUMA XC.
As we age, our faces become slimmer as a result of volume loss in deep fat compartments. Dermal filler administration has gained popularity in recent years, driven largely by improved safety and aesthetic outcomes. A report by Fortune Business Insights predicts the dermal filler market to grow to $6 million by 2026.
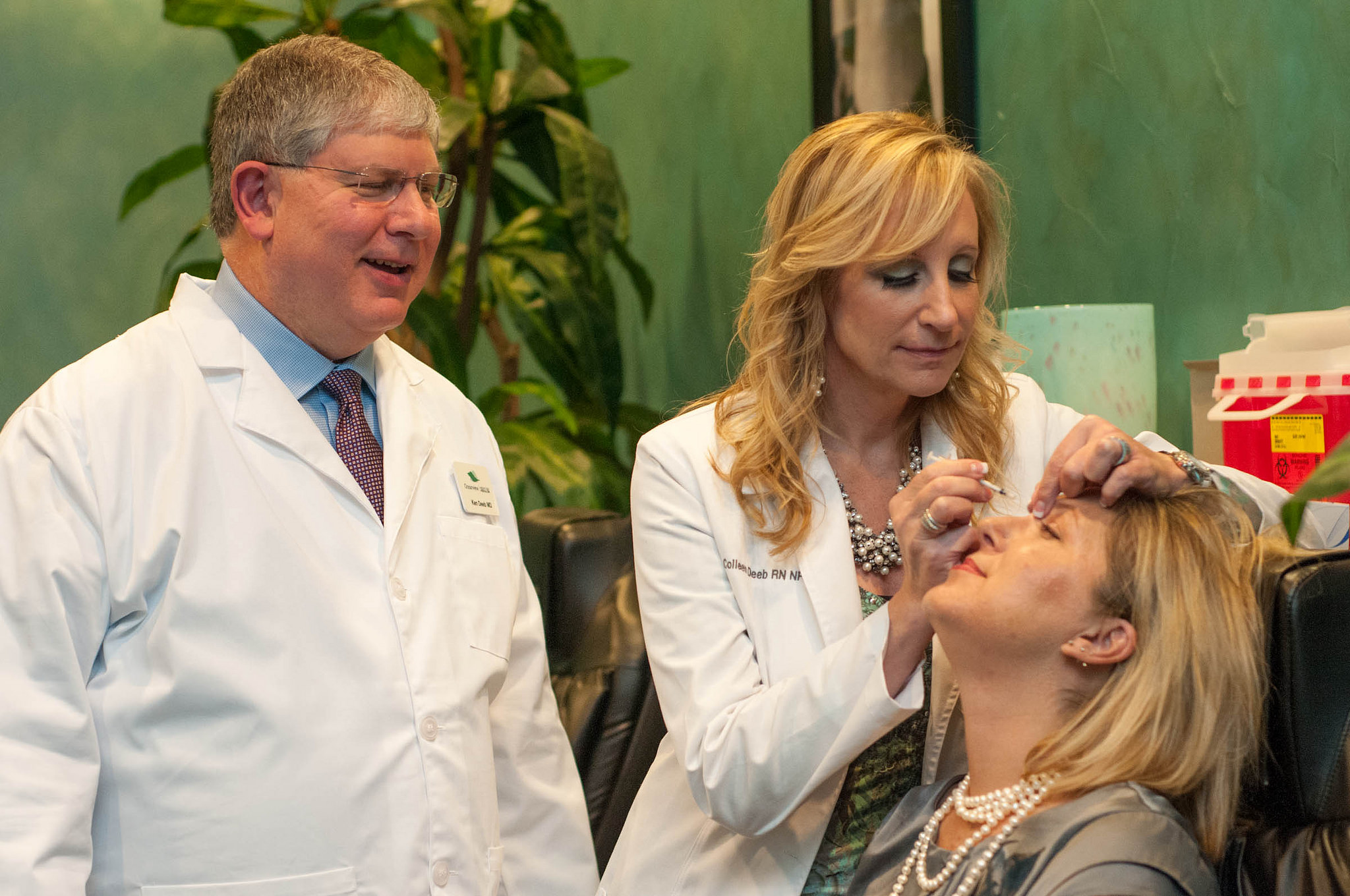
The Juvéderm VOLUMA XC is a hyaluronic acid gel-blend which helps correct age-related volume deficit cheeks for adults over the age of 21. Until now, all Juvéderm fillers were administered by needle, which could be painful for some patients.
The use of a cannula — a thin, flexible tube with a rounded tip — could be a welcome alternative for patients and physicians.
“As a physician, I have used the Juvéderm collection of fillers for 13 years, so I am thrilled that the FDA has approved the use of cannula with Juvéderm VOLUMA XC for mid-face volume deficit,” said Dr. Dee Anna Glaser, a board-certified dermatologist in St. Louis, in a statement. “With this latest approval, I have another effective option to provide volume and contour in the mid-face area. I can tailor my treatment approach for each patient while safely providing the aesthetic outcomes they wish to achieve,”
Glaser is also a clinical trial investigator who led a non-inferiority trial comparing needle versus cannula delivery of VOLUMA XC in 60 participants. The researchers found that all participants were satisfied with the outcome and safety of both methods of filler delivery.
In addition to less bruising and less pain, cannulas have been found to be more precise than sharp needles, contrary to popular belief. The perpendicular angle at which a needle typically pierces the skin can result in the spread of the filler material into superficial layers of the skin, also called retrograde flow. Therefore, the filler won’t be deposited at the tip of the needle. Cannulas, in contrast, can deliver fillers more precisely with less retrograde flow.
Ultimately, the precision, efficacy and safety of a dermal filler injection depends on the properties of the filler (viscosity, concentration of hyaluronic acid, particle size, etc.) and the technique use to administer it (angled vs. perpendicular, needle vs. cannula, size of needle or cannula lumen, risk of retrograde flow, etc.). While rare, a major risk of dermal filler injection is injection into a blood vessel, which could lead to the formation of blood clots and local tissue necrosis.
The Juvéderm collection of fillers showed a strong Q2, totaling $156.6 million in net revenues. Allergan revamped its Juvéderm campaign in late 2018, in hopes of developing the same brand loyalty as consumers have for Botox. Their new look targets millennials and Generation X using bright colors and edgy music.
Allergan’s FDA win comes just under a year after rival dermal filler maker, Galderma of Nestlé Skin Health, won approval for cannula delivery of its own hyaluronic acid-based dermal filler, Restylane Lyft.










Join or login to leave a comment
JOIN LOGIN