Yesterday, Scott Gottlieb, the Commissioner of the US Food and Drug Administration (FDA), announced the regulator would be laying out new guidance aimed at increasing the availability of generic combination products. These products, which combine a drug and a medical device, have featured prominently in the pharmaceutical pricing debate as true generics are often not available as alternatives.
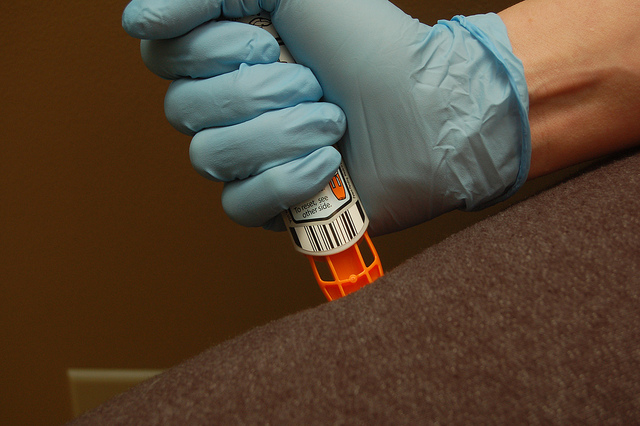
One of the best examples of this is Mylan’s EpiPen. The drugmaker has increased the list price for its two-pack of the anaphylaxis antidote over 500 percent over the last ten years. Despite this price hike, Mylan has enjoyed majority market share over this timeframe because patients had no other comparable option when it comes to buying an epinephrine autoinjector.
Many generics manufacturers, including Teva Pharmaceuticals, have tried to launch a less-expensive alternative to the EpiPen, but failed to have their combination product approved as interchangeable. Only Mylan has been able to release a true generic of its own product, which is released at a discounted price in hopes of appeasing regulators and patients.
“We have taken steps to provide industry with greater guidance on the types of information and analyses generic sponsors that want to copy a branded product, like an auto-injector or a metered dose inhaler, should submit to FDA to support approval of such products, even when the generic versions have some design differences relative to the branded drug they’re copying; and those design differences might correlate with different instructions for use of the two products,” said Gottlieb.
According to an article published in Forbes, the barrier to designing a generic EpiPen has been the FDA’s requirement that the autoinjector operate in exactly the same way as the original. Now, Gottlieb has announced that the regulator will be loosening that condition by allowing generics manufacturers to include different instructions for use, provided they can empirically prove that patients can understand them.
“The final guidance clarifies new policies related to these situations. This includes a principle we’re setting out that allows a generic product to have certain labeling differences from the branded product – if such labeling changes stem from permitted design differences,” said Gottlieb. “In other words, under this guidance, as long as the generic applicant is able to demonstrate with data, where appropriate, that differences in the design of the generic product do not affect the clinical effect or safety profile when the generic is substituted for the branded product, the generic product can be approved as a competitor to the branded drug where all other requirements for generic approval are met.”
The FDA Commissioner went on to say that the regulator was looking into ways to provide generic drugmakers with plans on how they could copy a complex drug two years before they’d be eligible for approval. They also plan on evaluating how combination products can be better evaluated on the basis of “sameness” with their branded counterpart, to speed their path to market.











Join or login to leave a comment
JOIN LOGIN