As clinical trials become more complex and more global, the amount of data they produce has grown exponentially. With that, the challenge becomes not only how to manage volumes of data from multiple sources, but also how to use it more effectively to identify, monitor and mitigate risk.
Regulatory agencies advise clinical trial sponsors to take a risk-based approach — which they call Risk-Based Quality Management (RBQM). A holistic approach centered on risk, they say, will lead to higher-quality trials. However, fully adopting their guidelines requires a sea change for clinical trial sponsors and their clinical research organization partners. Change isn’t easy in the pharmaceutical industry, but it’s necessary to accommodate the diversity of data sources and study designs.
Responding to the data deluge
Advances in genomic sequencing, increased use of wearable devices, and a move toward electronic patient-reported outcomes (ePRO), electronic informed consent (eConsent) and virtual or partially virtual trials (aka decentralized trials) have caused a data deluge. This increase in data has overwhelmed researchers and thrown a wrench in traditional risk-based monitoring.
Recognizing that clinical trials have grown too complex and expensive, the U.S. Food and Drug Administration and the European Medicines Agency started recommending a risk-based approach to monitoring in 2013. By designing a trial with risk in mind from the outset, clinical trial sponsors can improve efficiency, control costs and help ensure patient safety.
Today, RBQM is incorporated as a Good Clinical Practice expectation by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). The latest update, ICH E8 (R1), issued in May 2019, provides direction to help sponsors better manage, monitor and report data. While the guidelines raise as many questions as answers, they are a positive step toward changing the way pharmaceutical companies run clinical trials.
What is risk-based quality management?
RBQM is a system for managing quality throughout a clinical trial. RBQM is data-driven but can involve manual and automated tools. An RBQM strategy must be flexible to adapt to a changing, evolving environment.
RBQM involves the following key principles:
- Quality by Design (QbD). ICH describes QbD as a systematic approach to evaluating, understanding and refining drug development to focus on what affects critical quality attributes.¹ That knowledge helps sponsors establish a strategy to facilitate continual improvement and innovation.
- Risk-Based Monitoring (RBM). An RBM plan manages important risks to study participants and data quality by taking advantage of available technology.2 RBM focuses on preventing or mitigating important and likely risks to data quality.
- Trial-Specific Monitoring Activities. “On-site monitoring with high SDV [source data verification] is not the only way to ensure patient safety,” said Mike Arlotto, founder and CEO of Remarque Systems, a clinical trial quality-management platform provider. “When you design a protocol with risk in mind, you can create a monitoring plan based on those risks.”
A monitoring plan may include a combination of on-site monitoring and off-site, which includes monitoring through appointed committees and/or centralized monitoring. Research shows this type of trial-specific, risk-based monitoring ensures greater overall study quality than routine visits to sites with 100% SDV.3
The value of centralized monitoring
While in-person, on-site monitoring has advantages — for instance, the monitor can identify data-entry errors as well as assess whether site staff are following required protocols — centralized monitoring allows sponsors to evaluate data from multiple sites and sources remotely. Centralized monitoring can be used instead of or in addition to traditional site monitoring.
“Centralized monitoring has the potential to enhance insight, improve efficiency and optimize the integrity and accuracy of study data,” said Arlotto. “Issues such as the incorrect completion of assessment forms or non-compliance with the study drug — both of which can result in a total loss of data and potential cancellation of the trial — can be caught more quickly than they would using only an on-site monitoring approach.”
Why technology plays a leading role
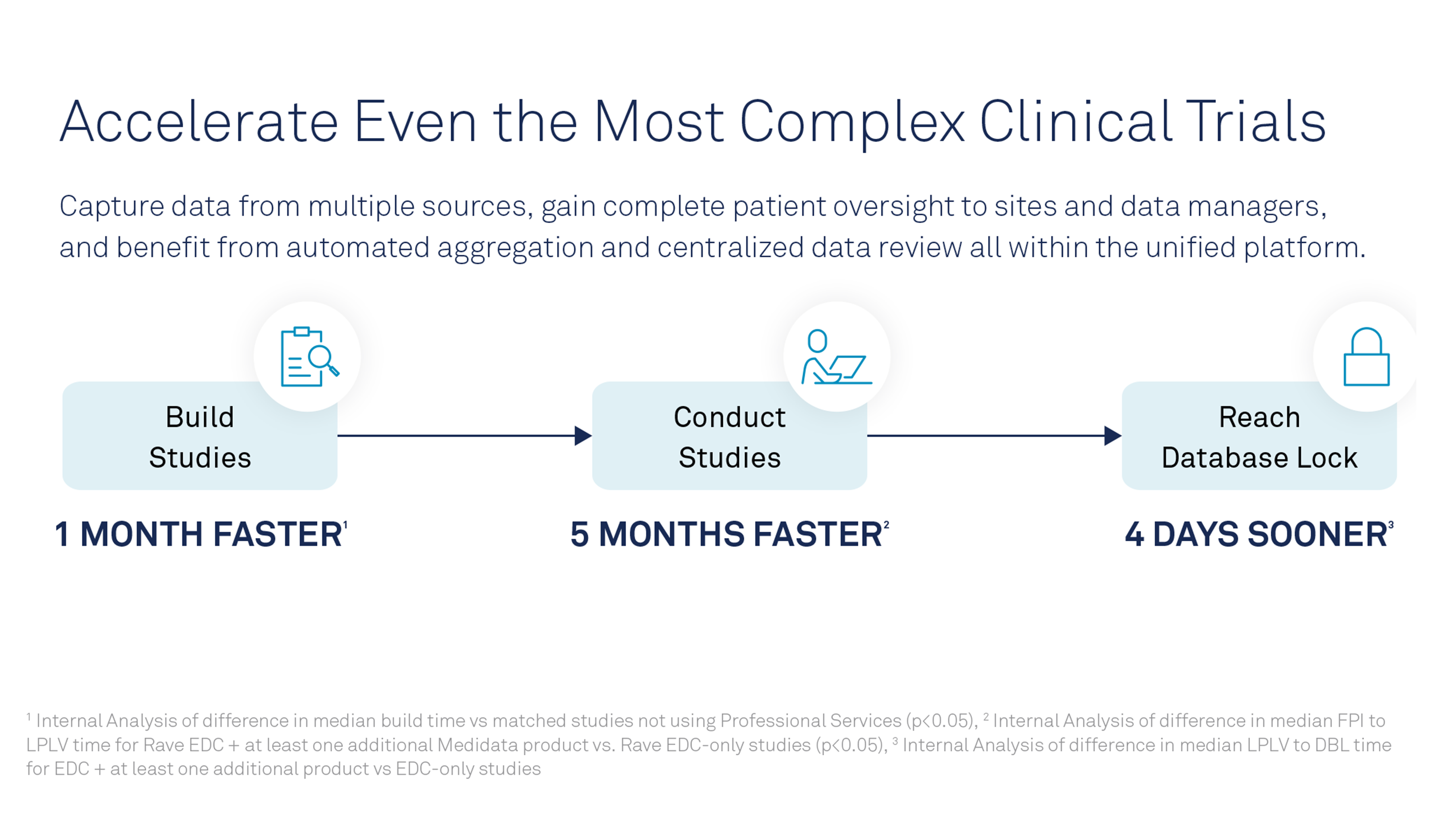
Incorporating centralized monitoring — a key component of RBQM — requires a shift in resources. It starts with updating processes to identify key risks early in the clinical trial and developing a plan to address them. It continues with training and retraining staff so they understand the RBQM strategy and follow a predefined process. It also requires technology that can accommodate large volumes of data.
The ideal platform can integrate data from multiple sources and systems. Central monitors must view electronic data capture, clinical trial management systems, lab data and data from interactive-response technology from a central platform. The platform should integrate this and other types of data, such as electronic medical records, ePRO, eConsent and wearables. It should also allow monitors to view this collective data in context with the risks affiliated with the trial.
Analytics capabilities allow sponsors to view trends within and across sites. If one site experiences a hiccup, a sponsor may need to investigate that site. If many sites experience the same hiccup, the sponsor may need to adjust its protocol. Conversely, analyzing a well-performing site may reveal insights to help other sites improve.
Analytics and machine learning also help sponsors develop mitigation plans that address quality issues. Viewing data in real-time helps sponsors spot errors quickly and take action, preventing minor issues from escalating into ruined study data or patient safety issues.
“Regulators want to know you’ve taken a risk-based approach to managing a study. They want to know the risks you’re going to manage, and they want to see documentation that you’ve performed the risk process,” Arlotto said. “It’s going to take a fundamental change in people, processes and technology to meet those expectations. It can be done successfully with a comprehensive platform.”
To learn more about clinical trial management systems, click the link to register for this free webinar.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.
References:
1 Guidance for Industry: Q8(R2) Pharmaceutical Development.
2, 3 Guidance for Industry: Oversight of Clinical Investigations — A Risk-Based Approach to Monitoring.









Join or login to leave a comment
JOIN LOGIN