Update (March 11, 2019):
Just three weeks after its launch in mid-February, over 37,000 Wixela-Inhub prescriptions have been filled – capturing almost a quarter of the market share for asthma products, according to IQVIA data. While Advair manufacturer, GSK, had anticipated generic competition, Mylan’s strong launch might potentially dent their long-term sales.
Originally published February 5, 2019:
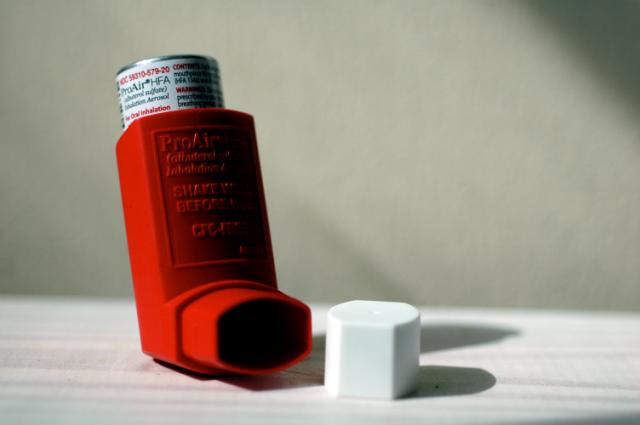
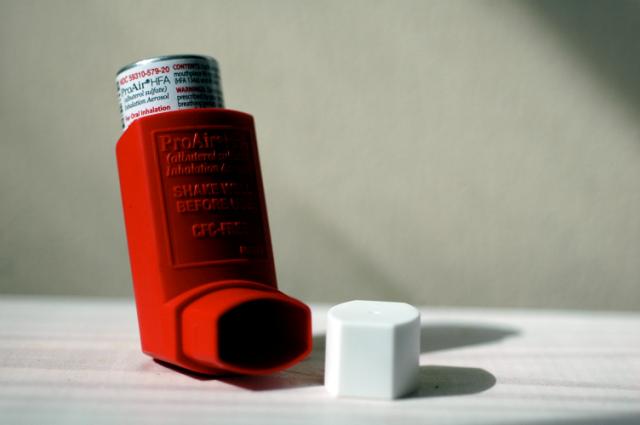
Mylan’s combination inhaler received US Food and Drug Administration (FDA) approval last week, becoming the first generic version (and newest rival) of GlaxoSmithKline (GSK)’s Advair.
Wixela Inhub is intended for the treatment of asthma and maintenance of chronic pulmonary obstructive disorder (COPD). The active ingredients are fluticasone propionate, a corticosteroid, and salmeterol inhalation powder, a long-acting beta-agonist. Together, the combination drug decreases inflammation in the airways and opens them up to make it easier to breathe.
Approximately 26 million people are living with asthma according to a Centers for Disease Control and Prevention (CDC) report in 2016, and COPD was deemed the third leading cause of death in the US in 2014.
“Asthma and respiratory specialists and primary care providers welcome this generic alternative to benefit many patients with asthma and COPD,” said Dr. Edward Kerwin, a clinical investigator on the Wixela Inhub clinical program. “We have waited for years for generic inhalers to emerge in respiratory medicine.”
Advair, the brand version by GSK, faced almost no competition since its approval in 2000, raking in sales of over $4 billion ending in November 2018. Ranging from $170-$270 depending on dose, it is no wonder that patients are eager for a cheaper alternative. Mylan has not released any pricing information, but we can expect a range of costs for the three approved strengths.
Formulating a safe and effective bioequivalent combination inhaler hasn’t been easy. Because inhalers are a combination of pharmaceutical drugs and medical device, they must meet more regulatory requirements than an oral drug. The FDA scrutinizes clinical efficacy data, safety data, as well as device manufacturing and packaging processes.
Several generic drug manufacturers faced regulatory setbacks that stopped their copycats from entering the market. In 2017, Teva received regulatory approval for AirDuo RespiClick– not a direct copycat of Advair, but one containing the same active ingredients at a lower dose.
“We’ve long been confident in the science around this product and are proud of the dedication of our scientific teams to bring Wixela Inhub to market,” said Mylan President, Rajiv Malik. “This complex product required a rigorous research and development program spanning over a decade and close collaboration with FDA to define the regulatory pathway.”
The FDA plans to release guidance documents to aid pharma companies in bringing complex generics to market, according to Anna Abram, FDA’s Deputy Commissioner for Policy, Planning, Legislation and Analysis.
The approval of Wixela Inhub is a breath of fresh air for Mylan, who faces heavy competition in the epinephrine injector market. On the other hand, GSK might only face short-term drops in sales, as they bolster their oncology profile with a recent partnership with Tesaro.





Join or login to leave a comment
JOIN LOGIN