In the US, the current term for a new drug patent is 20 years, ideally giving pharmaceutical companies two decades to make the most from a new drug launch, and hopefully recoup their development costs. However, in an effort to protect a company’s R&D investment, drug patents are often applied for before a pharmaceutical candidate even enters clinical trials. As clinical trials can take upwards of ten years to complete phases I to III, pharmaceutical companies may only benefit from half their original patent term by the time the drug gains US Food and Drug Administration (FDA) approval.
Once a drug begins to reach the end of its patent period, it’s vulnerable to being scooped up by generic drugmakers who seek to develop a copycat version of the medication. The FDA requires that generic versions of branded medications be chemically identical to their reference product, in addition to showing the same efficacy, safety and mode of action as the original drug.
While generic competition on the market helps to regulate drug costs and provide alternatives to patients, it inevitably eats into sales of branded medicines. According to John Phillips, a senior associate at Coopers & Lybrand Consulting, the first generic version of a drug to hit the market will command 80 percent of the generic market share.
Pay-for-Delay
Over the years, pharmaceutical companies have taken a variety of approaches to help mitigate the influence of generic launches on market share, especially for those increasingly rare blockbuster drugs. So-called pay-for-delay deals, in which innovator companies pay generic drugmakers to postpone the launch of their copycat drug, have become somewhat common in the pharmaceutical industry.
While these deals are not technically illegal in the US, the Federal Trade Commission (FTC) has been quite vocal about their disapproval of the practice. According to the FTC, pay-for-delay deals cost consumers $3.5 billion annually, as patients are stuck paying higher prices for branded drugs for longer periods of time.
In an effort to curb these deals, regulators outside the US – including the UK’s Competition and Markets Authority (CMA) – have started to crack down on anticompetition. Last year, the authority fined GlaxoSmithKline £37.6 million for engaging in a number of pay-for-delay deals with generics companies for its antidepressant drug, Seroxat.
As previously mentioned, it’s particularly advantageous to be the first to file a generic drug application after its branded counterpart has gone off-patent. Under the Drug Price Competition and Patent Term Restoration Act of 1984 – more commonly known as the Hatch-Waxman Amendments – this first-filer is granted a 180-day exclusivity period by the FDA after its generic product is launched.
During this six month period, the FDA is not permitted to approve any other generic versions of the reference product. As such, the generic drugmaker benefits from high revenues in the absence of any other generic drug competition.
Here’s where things get interesting: in another attempt to delay a generic drug’s market launch, the brand name pharmaceutical company can launch a generic version of their own drug. Known as an authorized generic, this tactic allows the branded drug manufacturer to compete with a less-costly generic alternative in the 180-day exclusivity period.
Authorized Generics
In 2015, market research firm Cutting Edge Information, found that companies who implemented an authorized generics strategy saw over 5,000 percent return-on-investment (ROI). Although they say that this is not the most widely-used lifecycle management strategy, it is one of the best ways for pharmaceutical companies to maintain profits after the loss of market exclusivity.
Authorized generics are identical to the company’s branded product in terms of both active and inactive excipient ingredients; the only thing missing is the brand name and packaging that comes along with it. In comparison, unauthorized generics are only required to contain the same active ingredient as its reference product.
Proponents of authorized generics say that the practice introduces beneficial competition into the market, lowering generic prices for consumers in the long run. A 2011 report published by the Federal Trade Commission (FTC) found that generic prices were four to eight percent lower when an authorized generic was released during the 180-day exclusivity period, compared to the absence of any authorized generic competition.
While the introduction of these authorized generics certainly has a negative effect on the generic pharmaceutical company’s bottom line, the FTC found that the competition has no measurable long-term effect on reducing incentives for these generics firms. The FTC’s conclusion is in contrast to some industry opponents of authorized generics, who believe that the practice reduces competition.
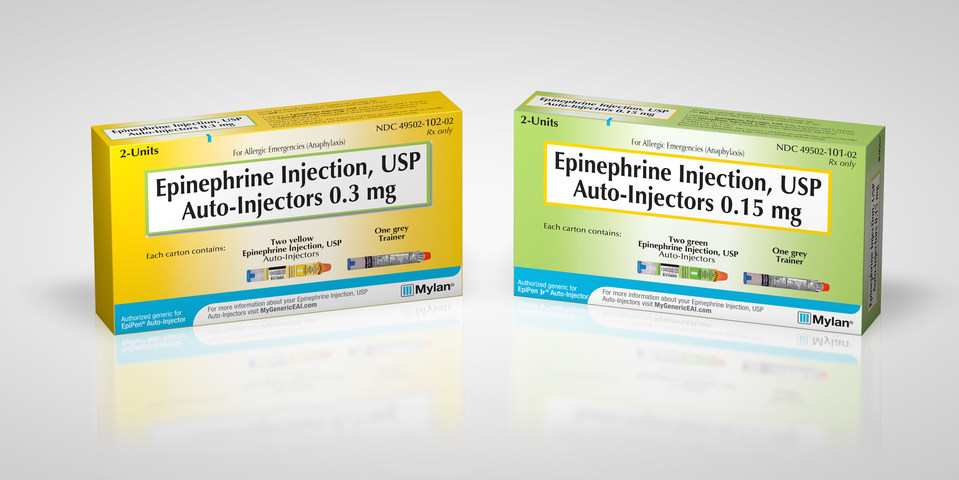
Mylan’s EpiPen
Most recently, pharmaceutical company Mylan released an authorized generic version of its EpiPen epinephrine auto-injector. Mylan had been facing criticism over its decision to significantly increase the cost of the EpiPen, a medical device with close to 95 percent of the market share.
While the case of the authorized generic EpiPen is unique in that it was introduced before true generic competitors hit the market, the launch still serves to keep Mylan competitive in the face of rival devices. It’s price of $300 for a two-pack has also set the benchmark for generic epinephrine auto-injectors.
The launch of Mylan’s authorized generic EpiPen has done little to change the views of critics. Rachel Sachs, a law professor at Washington University in St. Louis, wrote an exhaustive post about the move on the Harvard Law Blog.
“I wonder whether their generic will be fully substitutable by pharmacists, a question which may depend on the state laws involved (and which Mylan has lobbied to influence),” wrote Sachs. “I wonder whether the EpiPen comes with training or other easy-to-use tools that will be missing from the generic, and as long as Mylan provides copay coupons only for the branded product, parents facing equivalent copays in either situation will choose the branded EpiPen.”
The launch of authorized generics is a practice which will likely continue to be employed by pharmaceutical companies in the face of patent expiry. While the move remains controversial, industry regulators maintain that authorized generics are beneficial for consumers.
How do you think the launch of authorized generics affects the pharmaceutical industry? Share your opinion in the comments section below!











Join or login to leave a comment
JOIN LOGIN