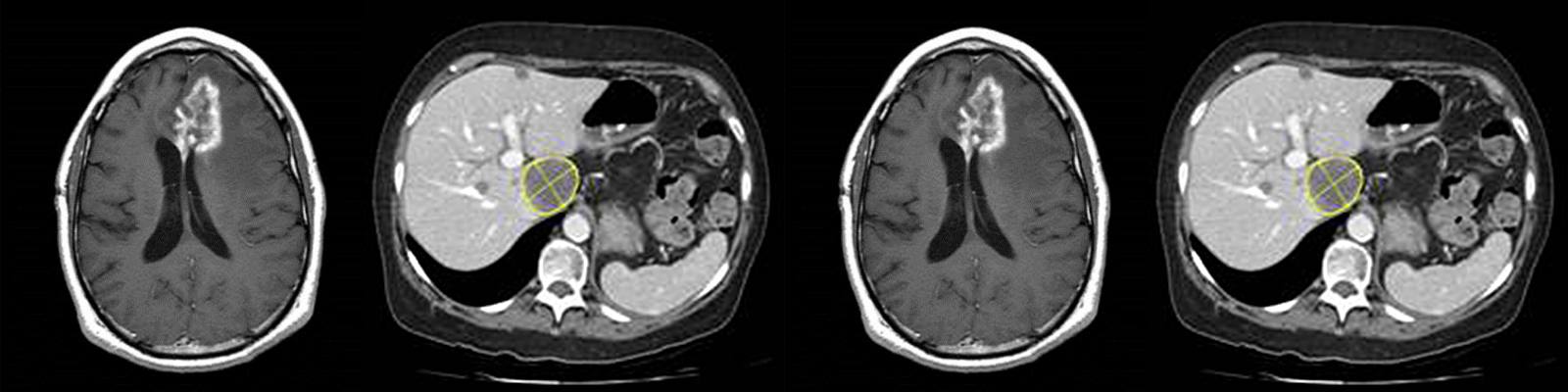
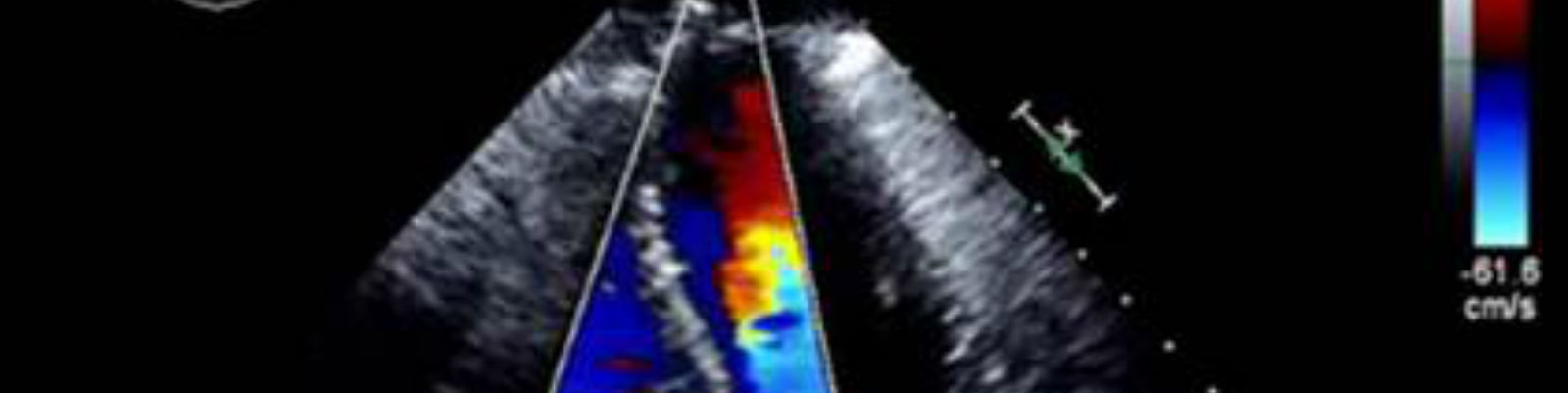
Medical imaging provides valuable efficacy and safety information in support of oncology clinical development. As biopharmaceutical companies continue to identify ways to improve, streamline and speed up product development, medical imaging plays a more significant role. Moreover, well-conceived and selective medical imaging approaches help control the ever increasing cost, complexity and duration of oncology clinical trials.
In this webinar, industry experts Sandra Chica, MD and Polina Voloshko, MD aim to describe novel medical imaging approaches and methodologies to help sponsors maximize clinical trial efficiency along the development pathway. Special emphasis will be placed on the assessment of tumor response and cardiac safety to support internal decision-making.
Webinar objectives include:
- Understand the varied roles of medical imaging to assess efficacy and safety in Oncology clinical trials
- Recognize the value of selective and tiered efficacy and safety medical imaging approaches for the various Oncology clinical development phases
- Appreciate real-world examples of how customized medical imaging approaches can benefit Oncology clinical trial conduct and execution
Speakers

Sandra Chica, MD, VP of Medical Imaging, BioTelemetry Research
Dr. Chica oversees the medical/scientific and operational direction of the company’s Imaging services across multiple therapeutic areas. As a board certified radiologist with 15 years of Pharmaceutical industry and clinical research experience, Dr. Chica brings both scientific and business expertise to enhance the company’s Imaging offering. Before joining Cardiocore, Dr. Chica was Novartis’ Global Head, Imaging, Oncology Clinical Development, and prior to Novartis, she served as the Senior Director of Medical Affairs and Head of Oncology for Perceptive Informatics (now PAREXEL Informatics).

Polina Voloshko, MD, Chief Medical Officer, BioTelemetry Research
Dr. Voloshko has nearly 25 years of experience in ECG, ECHO and Holter research. Prior to joining the company, she was VP of Cardiovascular Clinical Services at the Ischemia Research and Education Foundation and Gentiae. Previously, Dr. Voloshko served as a research fellow at the University of California San Francisco (UCSF) and Chief of Cardiology at Riga City Hospital in Riga, Latvia, an affiliate of the Latvian Medical University. Board-certified in cardiology and internal medicine, Dr. Voloshko received her MD, magna cum laude, at the First St. Petersburg Medical School in Russia.
Who Should Attend?
This webinar will benefit medical and non-medical professionals in the biopharmaceutical industry, especially those supporting Oncology, Immuno-Oncology and/or Hematology drug development with roles in:
-
Clinical Research
-
Clinical Development
-
Medical Affairs
-
Clinical Operations
-
Project Management
-
Regulatory Affairs
Xtalks Partner
BioTelemetry
As the research division of BioTelemetry Inc. (Nasdaq: BEAT), BioTelemetry Research, formerly Cardiocore, provides one of the world’s largest clinical data networks. Our broad range of Cardiac and Imaging services support both safety assessments and efficacy evaluations across all major therapeutic areas, through all phases of clinical trials, in every global region.
BioTelemetry Research offers a full range of centralized clinical trial testing modalities for both safety and endpoint evaluation. Cardiac includes ECG, Holter, TTM, MCOT, ECHO, and ABPM/BP. BioTelemetry Research provides Imaging services across therapeutic areas, including Oncology, CNS, Musculoskeletal, and Cardiovascular. Supported Imaging modalities include MRI, X-Ray, PET/CT, Bone Scintigraphy, and others.
Media Partner
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account