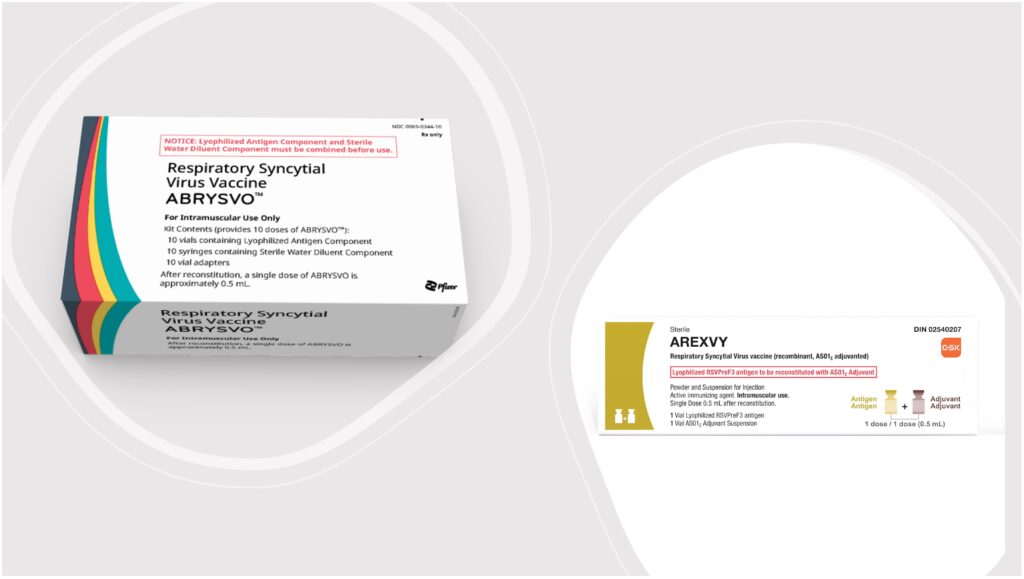
GlaxoSmithKline’s (GSK) Arexvy and Pfizer’s Abrysvo are the first-ever approved vaccines against respiratory syncytial virus (RSV). The vaccines received approvals from the US Food and Drug Administration (FDA) in May 2023 for the prevention of RSV infection.
With approvals for adults aged 60 and over, and Abrysvo’s additional nod for maternal use, the vaccines are pivotal advancements in RSV prevention, particularly in vulnerable populations like older adults and infants.
Their introduction comes at a crucial time with a highly active viral climate that has included influenza and COVID-19 along with RSV for the past couple of respiratory virus seasons.
Like the influenza and COVID vaccines, the aim of the new RSV vaccines is to reduce severe respiratory complications associated with RSV and related hospitalizations.
Take a closer look at how the vaccines compare with respect to their efficacy, side effects and market performance so far.
Type of Vaccine
Arexvy and Abrysvo are both recombinant protein subunit vaccines. Specifically, they are stabilized prefusion F subunit vaccines that target the fusion protein of the virus, which helps the virus enter and infect cells.
The difference between the two is that Abrysvo is a bivalent vaccine containing equal amounts of stabilized prefusion F antigens from the two major RSV subgroups: RSV A and RSV B.
Another difference is that Arexvy contains an adjuvant while Abrysvo does not.
Number of doses
Both Arexvy and Abrysvo are given as a single dose.
Approvals
Arexvy and Abrysvo are both approved for the prevention of RSV infection in adults 60 years of age and older. Abrysvo has an additional approval as a maternal vaccine for pregnant individuals at 32 through 36 weeks gestational age to prevent lower respiratory tract disease (LRTD) and severe LRTD caused by RSV in infants from birth through six months of age.
The current RSV vaccines were developed to help prevent RSV-related complications to prevent hospitalizations.
Efficacy of Arexvy versus Abrysvo
In Phase III trials, Arexvy was shown to offer 94.6 percent protection against severe RSV pneumonia and 72 percent efficacy against RSV acute respiratory infection in adults aged 60 and over with one dose.
In a Phase III study, Abrysvo demonstrated a 67 percent efficacy in preventing RSV infections with at least two symptoms in adults aged 60 and above. It was 86 percent effective against more severe disease characterized by three related symptoms.
As a maternal vaccine, in a study involving 3,500 pregnant women, Abrysvo significantly reduced the incidence of RSV-related respiratory illnesses in newborns by approximately 82 percent within the first three months after birth and 69 percent within six months after birth compared to placebo. The risk reduction increased to about 91 percent when the vaccine was given between 32 and 36 weeks of pregnancy.
Price of Arexvy versus Abrysvo
While Arexvy and Abrysvo are priced similarly, Arexvy costs slightly less. Arexvy costs $280 per dose while Abrysvo is $295.
Side Effects
Common side effects of Arexvy and Abrysvo include fatigue, headache, pain at injection site and muscle pain. In pregnant individuals, side effects of Abrysvo also include nausea. The side effects are typically mild to moderate and temporary.
Related: GSK’s Arexvy Leads Over Pfizer’s Abrysvo in Year One of RSV Vaccine Market Battle
Initial Market Performance: Arexvy Takes the Lead
Commercially, in their first months on the market, Arexvy has taken the lead over Abrysvo, having earned $1.5 billion or £1.2 billion in 2023, while Abrysvo sales totalled $890 million.
So far Arexvy has outperformed Abrysvo commercially due to factors that include an impressive launch, high efficacy in older adults with underlying medical conditions and significant market share in retail sites.
Arexvy’s 94 percent efficacy and the focus on older adults, who represent the majority of RSV hospitalizations, have resonated well with healthcare providers. Additionally, Arexvy’s retail strategy captures a large portion of the immunization market for older adults.
Early advertising may have also helped Arexvy with its successful head start. GSK hit the ground running with ad campaigns to support Arexvy a few months before the shot was launched. This included one featuring basketball Hall of Famer Magic Johnson about RSV awareness in older adults. Recently, GSK Canada recruited hockey legend Wayne Gretzky for an RSV awareness and Arexvy vaccine ad. There are now over half a dozen TV ads for Arexvy. Abrysvo has fewer ads, including at least two TV spots that were launched in the fall of 2023 as well as one for maternal vaccination.
A third RSV vaccine may join Arexvy and Abrysvo as soon as this year, as Moderna is anticipating an FDA decision on its mRNA-based RSV shot in the first half of 2024.
The uptake of RSV vaccines has been low so far but is expected to grow as awareness builds around their importance in preventing RSV infection. According to the Centers for Disease Control and Prevention (CDC), as of February 16, 2024, 21.9 percent of adults 60 years of age and older were reported to have received an RSV vaccine.











Join or login to leave a comment
JOIN LOGIN