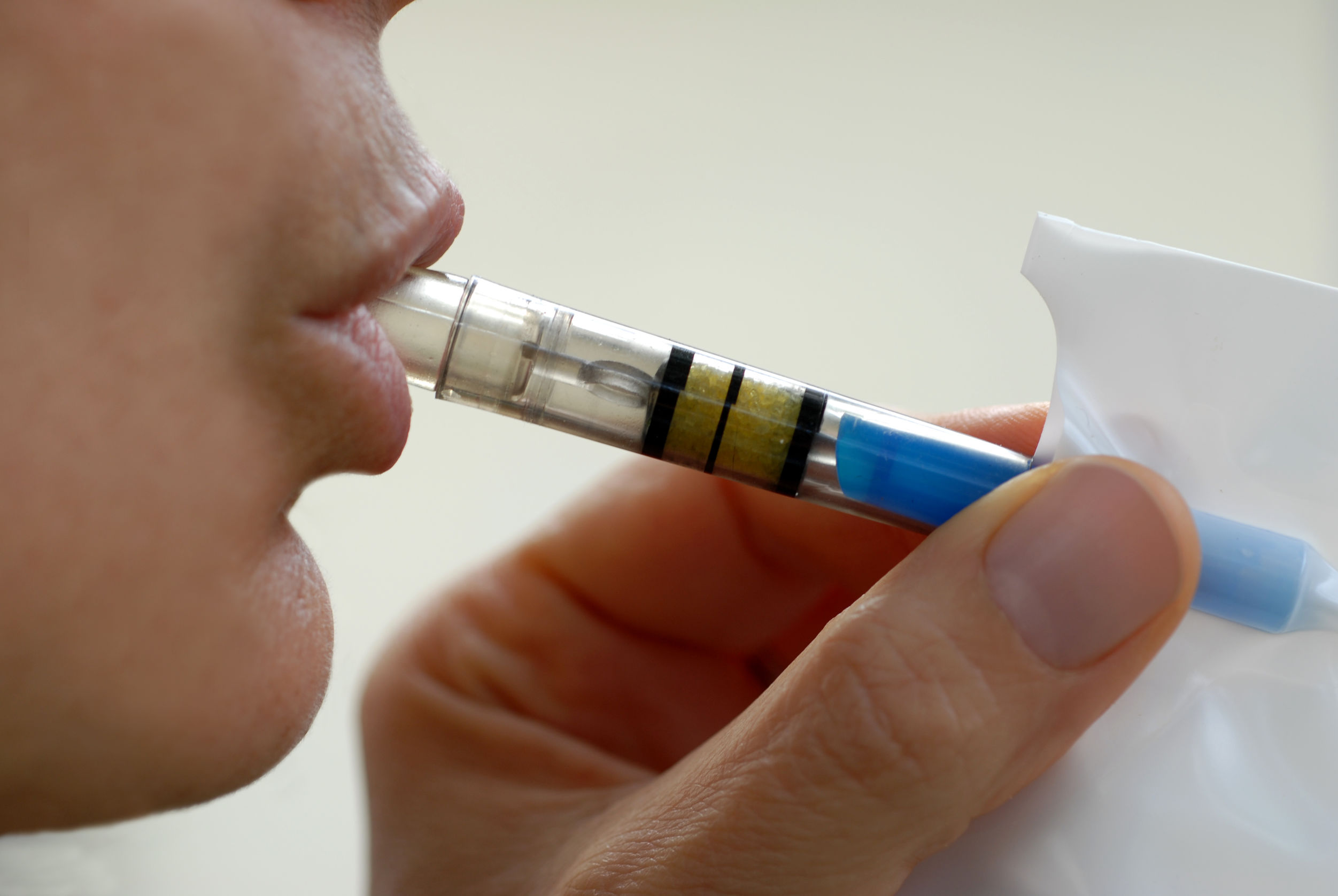
Earlier in April, several people took a pill that might have the ability to prevent colorectal cancer. These individuals are participants of a new pilot study launched by Check-Cap, a medical diagnostics company intent on developing the world’s first ingestible imaging capsule for colorectal cancer screening.
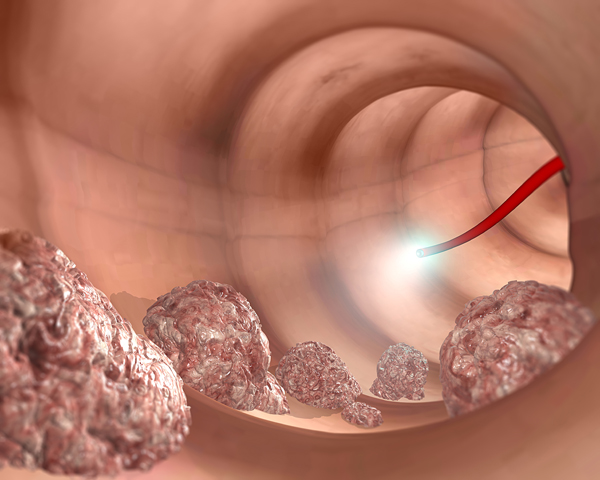
Colorectal cancer is one of the deadliest yet most preventable forms of cancer. Last year, over 880,000 people around the world died from this disease and the American Cancer Society estimates that 51,000 people in the US will die of it in 2019. If precancerous polyps or abnormal growths along the colon or rectum are found early, 90 out of 100 people are likely to live five years after the diagnosis.
Despite these odds, about one-third of the US population has never been screened for colorectal cancer. The daunting bowel preparation and fear of pain from the procedure are among the main reasons for why people may be hesitant to book an appointment for a colonoscopy.
“The potential addition of a new screening option that is capsule-based and does not require a bowel prep offers the promise of increasing our screening rates,” said Dr. Seth A. Gross, a gastroenterologist and Associate Professor of Medicine at New York University (NYU) Langone Health. The pilot study is taking place at NYU School of Medicine and may involve up to 45 patients.
The “C-Scan” system comprises three parts: a capsule that records spatial and structural information about the colon as it moves along; a tracking component that allows clinicians to follow the progress of the capsule as it moves through the patient’s colon; and a computer software that renders the imaging and positioning data into a 3-D map of the inner lining of the colon. From this map, clinicians can identify potential precancerous polyps and recommend next steps for the patient.
According to Check-Cap CEO, Alex Ovadia, the launch of the pilot study is already a big accomplishment.
“The pilot study initiation is a critical milestone on our path for developing and potentially commercializing the C-Scan system in the US,” he said. “We have also provided the FDA with the additional required information that allowed us to be granted with the full approval of the [Investigational Device Exemption (IDE)].”
The company is confident that this simple-to-use test will help catch colorectal cancers early and eventually reduce the overall incidence of the disease.
Check-Cap isn’t the first biotech company to tap the colorectal cancer prevention market. Last year, Canadian company Metabolomic Technologies Inc. began a $1.4 million (CAD) study evaluating PolypDx, a urine test for the detection and prevention of colorectal cancer. The Alberta-based company had previously signed several licensing deals to begin distributing the diagnostic test in the US.
If people prefer to take screening into their own hands, they could send their DNA to personal genomics biotech, 23andMe. Earlier this year, they received FDA approval to report on two common gene variants for hereditary colorectal cancer.
With these new approaches to colorectal cancer detection and prevention underway, potentially more people will get screened for this deadly but preventable disease.









Join or login to leave a comment
JOIN LOGIN