Companies developing oral biosimilar versions of injectable biologic drugs may have a better chance of capturing market share, according to a study conducted by research firm Frost & Sullivan and California-based biopharmaceutical company Rani Therapeutics. The collaborators say that their findings could not only help oral biosimilars gain momentum in the biologics market, but they could also help pharmaceutical companies protect their IP by developing their own oral version of large molecule biologics.
Humira (adalimumab) and Embrel (etanercept) are blockbuster biologics used to treat rheumatoid arthritis, Crohn’s disease and ulcerative colitis, among other autoimmune diseases. However, as these drugs are only available as injectable products, patient adherence is a concern. Currently, there is one biosimilar version of Embrel (Erelzi), and two biosimilars of Humira (Amjevita and Cyltezo) which have been approved by the US Food and Drug Administration (FDA), but all three of these biosimilars are injectable.
In their research, Frost & Sullivan surveyed over 600 individuals – 500 of whom were patients, with the remainder being physicians – to determine their preferred mode of administration for biologic drugs. They found that 88 percent of patients and 86 percent of physicians would prefer a once-daily Humira pill over the current bi-weekly injectable version of the drug.
“What is clear from our research is that patients and physicians are overwhelmingly in favor of replacing a syringe with a pill,” said Charlie Whelan, Director of Consulting for Frost & Sullivan’s Healthcare & Life Sciences practice. “There are millions of patients suffering from chronic diseases, and our research shows that convenience and reducing pain are hugely important to quality of life.”
The study also found that patient adherence to their medication schedule for Humira must be improved, with 62 percent of patients reporting missed doses. Worryingly, 86 percent of physicians say that patients often fail to inject Humira as prescribed. The physicians surveyed included both rheumatologists and gastroenterologists who overwhelming supported the idea that an oral version of these biologics could improve patient medication adherence.
“Humira is the #1 selling drug in the world, yet this research shows that its sales and AbbVie’s revenues could potentially be threatened by oral adalimumab because of a fear of needles and the associated lack of patient compliance,” said Mir Imran, Chairman & CEO of Rani Therapeutics. “Combine those challenges with patent expiration and the increasing threat of biosimilars, which are forecast to hit the market in the coming years, and it’s the perfect storm.”
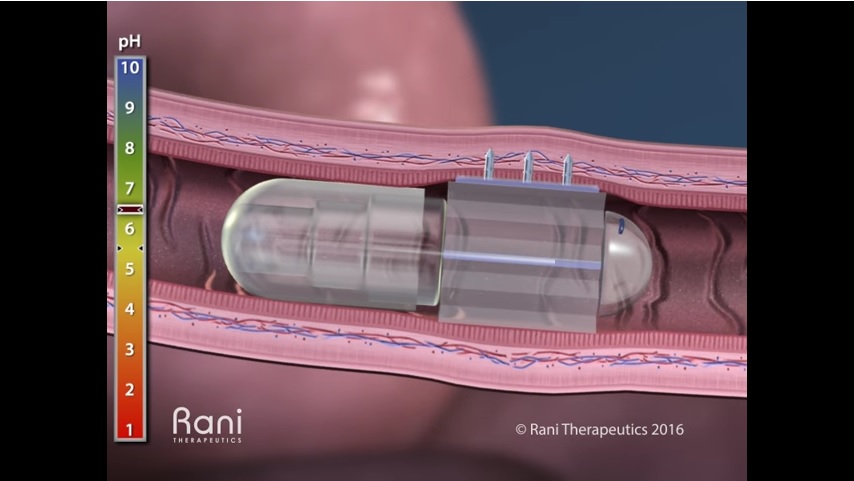
Rani Therapeutics had developed an oral delivery platform for many large molecule biologics which currently require injection. Backed by major players in the market, including Novartis, AstraZeneca, Shire Pharmaceuticals, Rani has raised $85 million to-date to support their technology.
Their patented oral delivery technology uses a device capable of triggering an injection in the intestine after the pill has traveled through the stomach. According to Rani, the drug is protected inside the acid-resistant capsule until it reaches the higher pH environment in the intestine. What’s more, they claim that bioavailability of the drug injected into the intestinal wall is as good, if not better, when compared to conventional subcutaneous injection through the skin.
“There has never been a better time for innovation in drug delivery,” said Imran. “Oral delivery of biologics is the next transformational event for pharmaceutical companies, and those that find alternatives to needles and differentiate based on drug delivery will be the big winners.”









Join or login to leave a comment
JOIN LOGIN