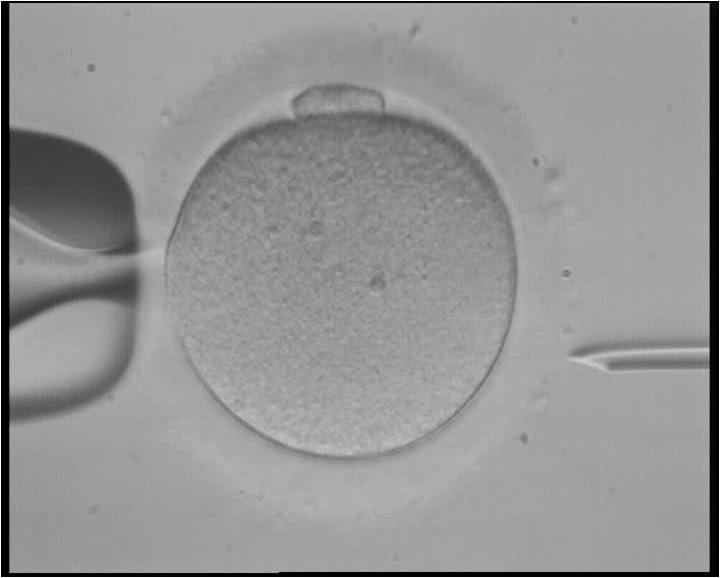
Using CRISPR gene editing, researchers at Oregon Health & Science University have successfully repaired a heart disease-causing mutation that can contribute to heart failure and sudden cardiac death. The details of the work were published in the journal Nature, and could represent a viable new method of preventing the mutation from being passed down from generation to generation.
Around one in every 500 individuals is affected by hypertrophic cardiomyopathy, making it one of the most common forms of genetic heart disease. The research represents the first time that this CRISPR method has been tested on human egg cells.
“Every generation on would carry this repair because we’ve removed the disease-causing gene variant from that family’s lineage,” said senior study author Dr. Shoukhrat Mitalipov, director of the Center for Embryonic Cell and Gene Therapy at OHSU. “By using this technique, it’s possible to reduce the burden of this heritable disease on the family and eventually the human population.”
According to the researchers, the gene editing technique could be applied to many other inherited diseases for which the disease-causing mutations have been identified. Since CRISPR is believed to be more precise when compared to other techniques, it could also improve the success rate of in vitro fertilization (IVF).
“If proven safe, this technique could potentially decrease the number of cycles needed for people trying to have children free of genetic disease,” said Dr. Paula Amato, associate professor of obstetrics and gynecology in the OHSU School of Medicine.
The researchers used donated clinical-quality healthy egg cells, as well as sperm with the hypertrophic cardiomyopathy mutation, to assess the effectiveness of CRISPR. By injecting the CRISPR enzymes and the mutated sperm cells into the egg simultaneously, Mitalipov and his colleagues were able to create an embryo that was free of the disease-causing mutation.
“This research significantly advances scientific understanding of the procedures that would be necessary to ensure the safety and efficacy of germline gene correction,” said Dr. Daniel Dorsa, senior vice president for research at OHSU. “The ethical considerations of moving this technology to clinical trials are complex and deserve significant public engagement before we can answer the broader question of whether it’s in humanity’s interest to alter human genes for future generations.”










Join or login to leave a comment
JOIN LOGIN