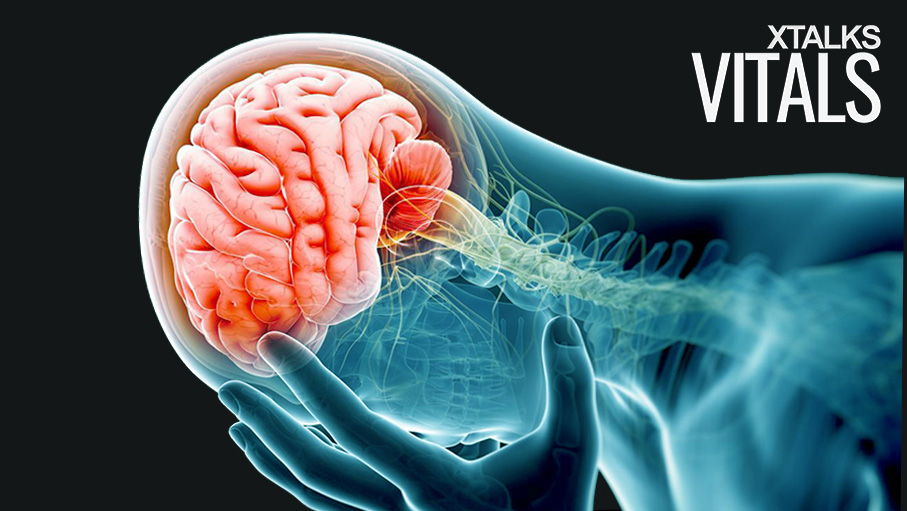
A group of neurosurgeons and engineers from the Washington University School of Medicine, in collaboration with researchers from the University of Illinois at Urbana-Champaign, have developed a bioresorbable brain sensor that eliminates the need for additional surgery to remove the device. The sensor – which monitors temperature and intracranial pressure – could be implanted to monitor patients who have sustained a traumatic brain injury (TBI).
Currently-available sensors are capable of measuring pressure on the brain following a traumatic brain injury, however they require removal surgery once they’ve performed their function. The researchers – whose paper was published in the journal, Nature – say their dissolvable sensor technology could also be applied to organ monitoring throughout the body.
A traumatic brain injury usually occurs after the head sustains some impact, which acts to change the normal function of the brain. Approximately 50,000 people die of a traumatic brain injury in the US each year.
Some patients who have sustained a traumatic brain injury experience amnesia and unconsciousness. According to Dr. Rory K. J. Murphy, a researcher at the Washington University School of Medicine, physicians are forced to use devices that “are based on technology from the 1980s” in order to accurately measure intracranial pressure following a traumatic brain injury.
“They’re large, they’re unwieldy, and they have wires that connect to monitors in the intensive care unit,” said Murphy. “They give accurate readings, and they help, but there are ways to make them better.”
Murphy points out that “a major hurdle has been that implants in the body often trigger an immune response,” which prevents implantable medical device technology from moving forward. In order to avoid the issue of biocompatibility, Murphy and his collaborators developed a medical device composed of polylactic-co-glycolic acid (PLGA) and silicone, which is capable of accurately measuring and transmitting temperature and pressure data.
The researchers have already tested the device in rat’s brains, and now plan to conduct a trial of the sensors using human patients. “With advanced materials and device designs, we demonstrated that it is possible to create electronic implants that offer high performance and clinically relevant operation in hardware that completely resorbs into the body after the relevant functions are no longer needed,” said Professor John A. Rogers, a researcher at the University of Illinois.
The device has been shown to be continuously effective for three days, at which time the device components are absorbed by the surrounding tissue. “You don’t have something in the body for a long time period, increasing the risk of infection, chronic inflammation and even erosion through the skin or the organ in which it’s placed,” said Murphy.
“The ultimate strategy is to have a device that you can place in the brain – or in other organs in the body – that is entirely implanted, intimately connected with the organ you want to monitor and can transmit signals wirelessly to provide information on the health of that organ, allowing doctors to intervene if necessary to prevent bigger problems,” said Murphy. “And then, after the critical period that you actually want to monitor, it will dissolve away and disappear.”










Join or login to leave a comment
JOIN LOGIN