In clinical research, diversity is more than a prerequisite for a successful drug — it’s a critical component that enhances the validity and applicability of clinical trial outcomes. The integration of diverse patient populations ensures that therapeutic interventions are effective across different demographics, reflecting real-world settings.
The need for adequate representation has given rise to several initiatives, the most notable and recent among which is the US Food and Drug Administration (FDA)’s “Diversity Action Plans to Improve Enrollment of Participants from Underrepresented Populations in Clinical Studies” draft guidance.
Diversity Action Plans have now moved from being a “nice-to-have” to a “must-have” in clinical trial designs.
Regulatory changes have been signaling the need for more inclusive clinical trials. With its recent draft guidance, the FDA has vitalized the importance of inclusive research, adding growing recognition to diversity’s role in clinical trials.

DRSc, MS, EMBA
Head of Regulatory Strategy &
Product Development
Fortrea

Senior Director, Regulatory
Strategy, Product Development
& Market Access Consulting
Fortrea
In this Xtalks Spotlight interview, we gathered insights from two key experts from Fortrea — Dr. Teresa Oblak, Senior Director of Regulatory Strategy, and Dr. Alicia M. Baker McDowell, Head of Regulatory Strategy. They shared their extensive knowledge of the importance and implementation of diversity action plans in clinical trials.
Dr. Oblak brings over a decade of experience in drug development, focusing on global clinical development programs and the development of Diversity Action Plans. Dr. McDowell, with an extensive background in global regulatory strategy and product development, has led numerous initiatives to register products worldwide.
What Are Diversity Action Plans?
“Diversity Action Plans include enrollment goals for underrepresented populations,” is how Dr. McDowell defined a Diversity Action Plan, adding that they provide a rationale for enrollment goals and a defined plan for achieving them (Figure 1).
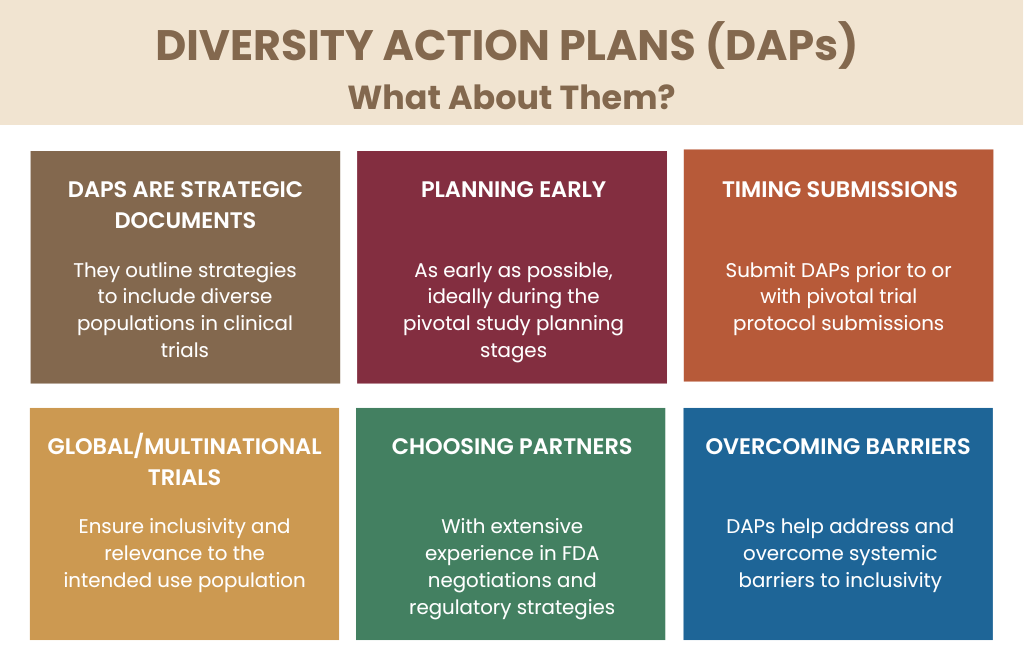
Diversity Action Plans must be a part of clinical research, especially following the passage of the Food and Drug Omnibus Reform Act (FDORA) of 2022, which legally requires these plans for all products heading into pivotal trials.
The planning process should begin as early as possible, ideally during Phase III or pivotal study planning stages, according to Dr. Oblak. Integrating diversity considerations early in the study design helps ensure that trial populations reflect the demographics of those who will ultimately use the treatment.
Submitting a Diversity Action Plan is the next significant milestone in a trial’s setup. “According to the legislation, a Diversity Action Plan is required to be submitted no later than the submission of a Phase III or a pivotal protocol. However, we have seen the FDA asking to submit these plans with an end of Phase II package with a SPA [Special Protocol Assessment] submission if a SPA is what you’re seeking for your pivotal protocol,” added Dr. McDowell, explaining that some sponsors prefer to provide the FDA with a more comprehensive view early in the process and to ensure timely integration.
Global Trials and the Complexities of Inclusivity
For clinical trials with a global or multinational reach, ensuring diversity poses additional challenges. “Diversity Action Plans should encompass the entire study population, not just participants enrolled in the US,” highlighted Dr. Oblak. While the goals and site selections need to align with US epidemiology, the global nature of many studies means sponsors must adapt to diverse populations across multiple regions. Dr. Oblak added, “Sponsors should be prepared to track their studies and relevant diversity goals for the US and from a global perspective.”
With extensive experience amassed in navigating diversity challenges, Fortrea’s team works across therapeutic areas and regions, ensuring that diversity goals are met not only in the US but in the broader global context of clinical trials.
“At Fortrea, we’ve developed over 40 Diversity Action Plans across various therapeutic areas and FDA centers,” Dr. McDowell notes. Through community engagement and thoughtful trial designs, Fortrea’s approach addresses barriers such as language, geographic access and local healthcare infrastructure.
Diversity Action Plans also promote trial designs that accommodate the specific needs of different patient groups, including flexible scheduling, transportation support and access to technology for remote monitoring.
A Diversity Action Plan Can Give Visibility to Systemic Barriers
When asked about the patient impact of Diversity Action Plans, Dr. Oblak emphasized the direct benefits, saying, “When a Diversity Action Plan is formalized, it’s the culmination of dynamic efforts to improve representation in clinical studies as well as enhance the strengths and generalizability of available evidence for the intended use population of a product.”
By removing systemic barriers, Diversity Action Plans ensure that diverse patient groups, particularly those historically underrepresented, can access trials. This includes identifying and addressing obstacles like geographic location, language barriers and awareness of clinical trial opportunities. Building relationships with community leaders and organizations also helps raise awareness and trust, encouraging greater participation from diverse populations.
In addition to improving trial access, Diversity Action Plans also ensure that the trial data is more reflective of real-world populations. This makes them more applicable to the right populations.
Dr. McDowell and Dr. Oblak present a compelling picture of the importance of inclusive and representative research. Diversity Action Plans are pivotal in this shift, ensuring that all patient groups have access to clinical trials and that the data generated is indeed representative of the population each drug or therapy will treat.
Increasing diversity in clinical trials is essential to ensuring that treatments are both effective and safe for a wide range of patient populations, ultimately leading to more equitable healthcare outcomes.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.






Join or login to leave a comment
JOIN LOGIN