People living with type 2 diabetes will soon have access to a new, needle-free diabetes treatment, thanks to the approval of the world’s first oral semaglutide tablet.
The US Food and Drug Administration (FDA) approval was granted to Danish drugmaker Novo Nordisk on September 20, 2019 for 7 mg and 14 mg doses of Rybelsus. The oral glucagon-like peptide (GLP-1) receptor agonist, used together with diet and exercise, is intended to improve blood sugar control in adults with type 2 diabetes.
Type 2 diabetes affects more than 30 million people in the US, according to the Centers for Disease Control and Prevention. There are numerous insulin-based and glucagon-based treatments available to patients, often coming in the form of daily or weekly injections. Novo Nordisk, one of the biggest producers of diabetes drugs in the world, will provide a new option for diabetics who would prefer a once-daily oral tablet.
“Novo Nordisk has a very long legacy of developing innovative injectable medicines for people living with diabetes and, with the approval of Rybelsus, we are now able to bring our innovation into the market for oral antidiabetics,” said Mads Krogsgaard Thomsen, executive vice president and chief science officer of Novo Nordisk, in a statement.
The company could take pride in the novelty of the first oral semaglutide tablet approved in the US while competitor pharma companies find ways to formulate their own. Oramed Pharmaceuticals also has an oral GLP-1 analogue in the works, but it may have a better chance at bringing the first oral insulin drug to market, having recently enrolled its last patient into a Phase IIb trial.
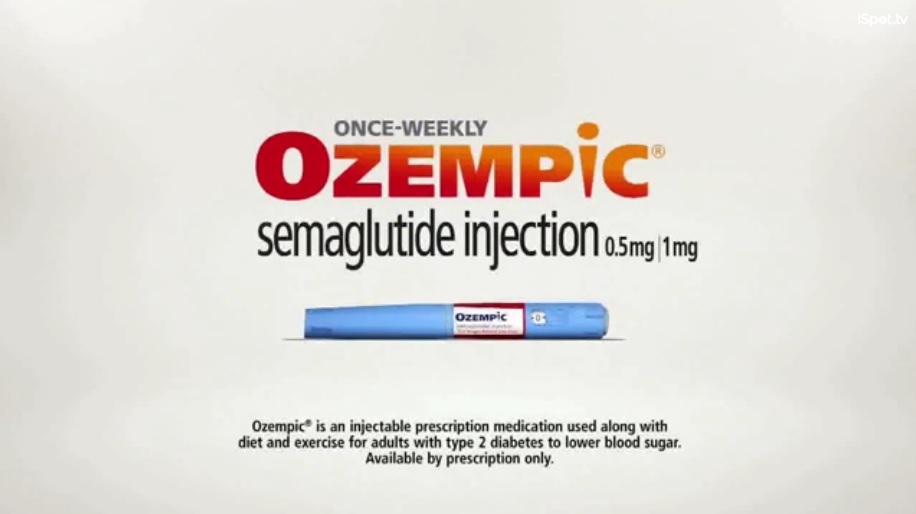
The cost of Rybelsus will play a critical role in Novo Nordisk’s success. The company’s injectable semaglutide, Ozempic, is hugely successful despite carrying a price tag of $800 per month. Novo Nordisk didn’t reveal Rybelsus’ price immediately, saying it would be priced at a “similar level as Ozempic.” The company also revealed that they will begin negotiating with pharmacy benefit managers and insurance providers to lower out-of-pocket costs when the product is set to launch at the end of 2019.
The high cost of insulin continues to concern patients, providers and legislators alike, and pharma companies are attempting to slash prices. Eli Lilly released a half-priced version of its branded insulin, Humalog, earlier this year at under $300 for five pens. However, a US Senator was quick to point out that this reduced-price generic was still too expensive, saying Eli Lilly is charging Americans four times more than what Canadians pay. In fact, a pharmacist in Windsor, Ontario told CBC that Americans come to his pharmacy about 15 times a month to buy cheaper insulin.
While Rybelsus might not be able to deliver in price, it will likely deliver in efficacy. Rybelsus works by slowing digestion and stimulating insulin secretion while lowering glucagon secretion. The drug was tested in over 9,500 adults with type 2 diabetes across at least 10 clinical trials. The FDA approval was granted based on these studies, which touted adequate safety data, plus significant reductions in blood sugar levels (measured by HbA1C) and body weight.
The oral semaglutide tablet comes with an FDA boxed warning, alerting patients of the risk for thyroid C-cell tumor, a condition that has been linked to long-term use of GLP-1 analogues. The European Medicines Agency and Japanese Pharmaceuticals and Medical Devices Agency might follow in the FDA’s footprints if they, too, sign off on Rybelsus.
Nonetheless, the emergence of the first oral glucagon-like peptide on the diabetes drug market is a positive sign for type 2 diabetes patients.
“Patients want effective treatment options for diabetes that are as minimally intrusive on their lives as possible, and the FDA welcomes the advancement of new therapeutic options that can make it easier for patients to control their condition,” said Dr. Lisa Yanoff, acting director of the Division of Metabolism and Endocrinology Products in the FDA’s Center for Drug Evaluation and Research, in a statement. “Before this approval, patients did not have an oral GLP1 option to treat their type 2 diabetes, and now patients will have a new option for treating type 2 diabetes without injections.”










Join or login to leave a comment
JOIN LOGIN