The FDA has granted clearance to Heidelberg Engineering’s Epithelial Thickness Module (ETM) integrated into the company’s Cornea App, which is a part of the Anterion ocular imaging platform.
The new module is expected to enhance precision in corneal health assessment, offering ophthalmologists a robust tool for more comprehensive and accurate evaluation of the corneal epithelium.
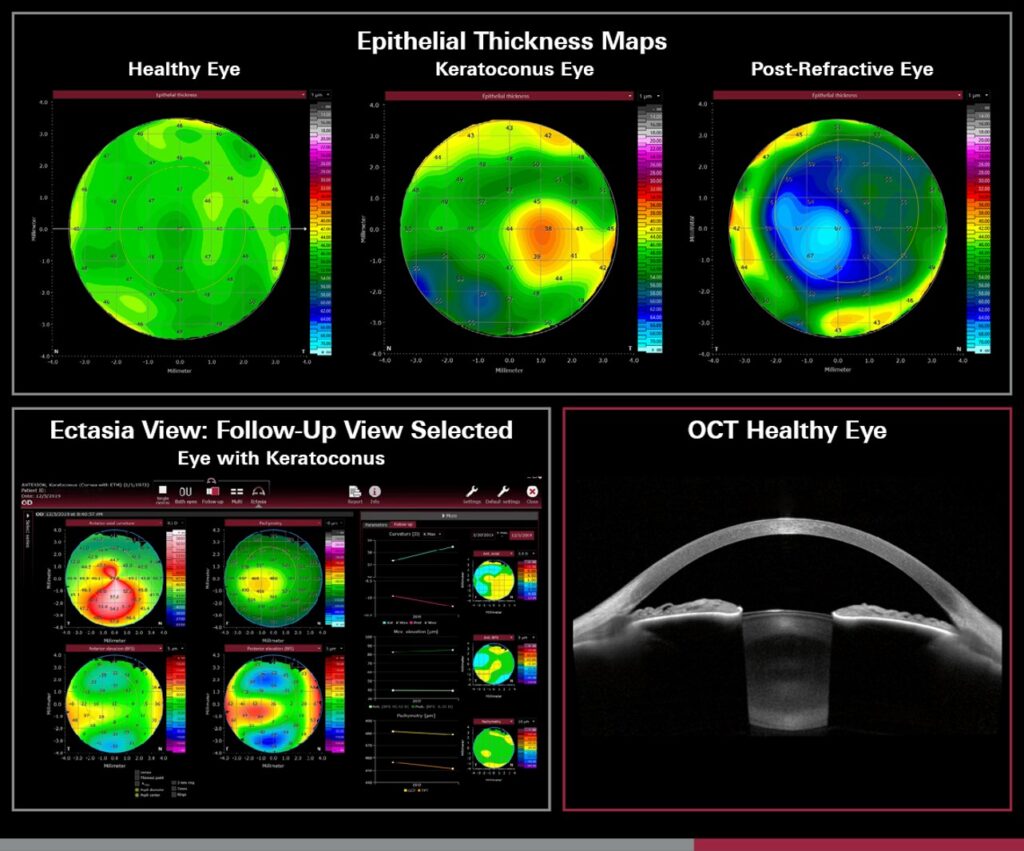
According to Heidelberg, the module provides eye care professionals with advanced tools for epithelial and stromal thickness mapping to deliver valuable data insights. With comprehensive parameters and detailed color maps, it is designed to enhance refractive surgery planning, support ocular surface evaluation, aid in the assessment of corneal ectasia and assist in a wide range of other corneal diagnostic applications.
ETM is available exclusively through the Anterion Cornea App.
The epithelial layer of the cornea plays a critical role in maintaining ocular surface integrity and visual acuity. Variations in epithelial thickness can indicate early stages of ocular conditions, such as keratoconus, dry eye disease and epithelial basement membrane dystrophy.
By enabling precise, high-resolution measurement of epithelial thickness across the entire cornea, the new ETM provides a means for detecting subtle changes that could otherwise go unnoticed.
Related: LumiThera’s Valeda Photobiomodulation System Gets FDA Authorization for Dry AMD
“With the FDA clearance of the Epithelial Thickness Module, we are excited to expand the capabilities of the Anterion Cornea App and provide eye care professionals with an even more robust toolkit for assessing their patients’ corneal health,” said Ram Liebenthal, General Manager of Heidelberg Engineering USA.
“From the beginning, our vision has been to provide precise data and reproducible measurements that allow clinicians to make informed decisions, enhance patient care and streamline workflows. This latest FDA clearance is a key milestone in fulfilling that mission.”
Anterion is a modular anterior segment imaging platform that combines biometry, intraocular lens (IOL) power calculation, corneal topography and tomography, anterior chamber metrics and high-resolution swept-source OCT (SS-OCT) imaging into a single device.
The ETM utilizes Anterion’s SS-OCT technology to generate high-resolution, detailed, cross-sectional images of the eye.
The SS-OCT technology employed by Anterion provides a wavelength of 1300 nm, ensuring deeper tissue penetration and higher-resolution imaging compared to conventional OCT systems. This allows for the accurate mapping of the epithelial layer with micron-level precision.
At the core of the Anterion platform is the imaging app, which is designed to deliver high-resolution images of the anterior segment to help clinicians diagnose and manage anterior segment alterations.
Anterion also now includes Ectasia View, a toolset designed to help clinicians assess and track ectatic changes — which involve distension or dilation of a duct, vessel or hollow viscus — in the cornea, including conditions like keratoconus. The feature consolidates critical corneal data from multiple visits into one dashboard for a streamlined overview of key tomographic maps and parameters to support efficient clinical decision-making.
Heidelberg says the added features and capabilities of the ETM and Ectasia View are “helping to transform daily clinical workflows, improve precision and enhance efficiencies for clinicians.”
Anterion has been commercially available in the US since 2024 after getting FDA clearance in October 2023. The ETM is scheduled for commercial release in February 2025.
Current Anterion Cornea App users can access the module through a straightforward software update. For new customers, the module will come pre-integrated with the Anterion Cornea App when they purchase it, providing an all-in-one solution.
If you want your company to be featured on Xtalks.com, please email [email protected].









Join or login to leave a comment
JOIN LOGIN