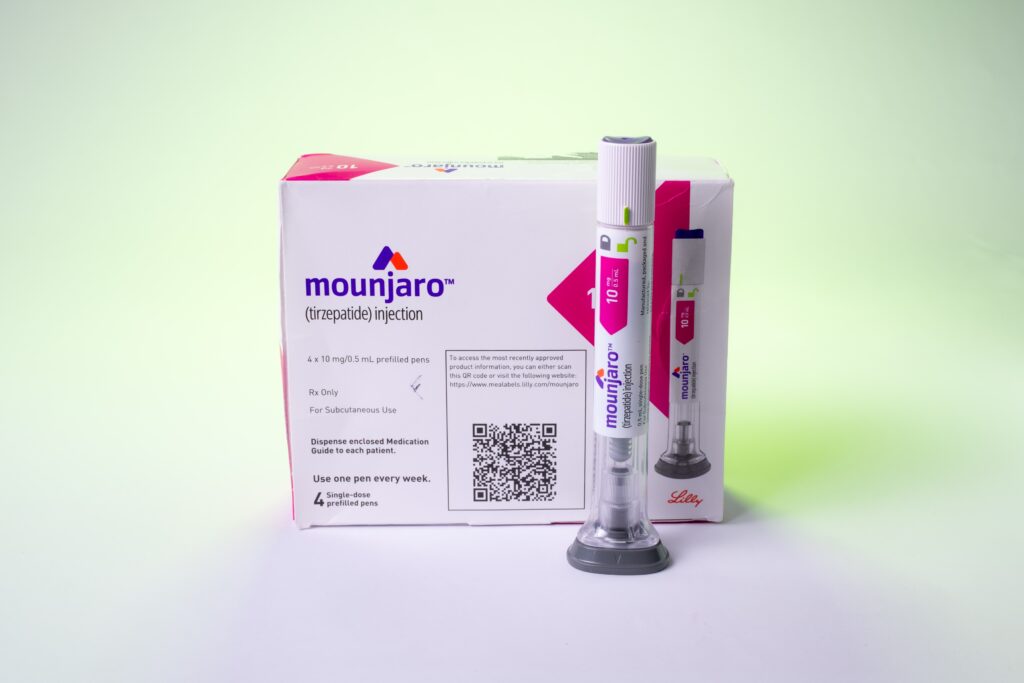
After a nearly two-year-long shortage, the US Food and Drug Administration (FDA) announced earlier this month that Eli Lilly’s GLP-1 diabetes drug Mounjaro (tirzepatide) is back on shelves along with weight loss version Zepbound.
A few days later, GLP-1 drug rival Novo Nordisk announced that four out of five dose strengths of its GLP-1 obesity drug Wegovy (0.5 mg, 1 mg, 1.7 mg and 2.4 mg) are now available in the US. However, Novo said it will continue to manage shipments of the drug’s 0.25 mg starter dose to “responsibly initiate patients on treatment.”
Novo is the maker of the wildly popular Ozempic and its weight loss counterpart Wegovy, both formulations of semaglutide. The drugs have been on the FDA’s drug shortages list since 2022.
With the massive popularity of GLP-1 drugs for diabetes and weight loss, the shortages were almost inevitable as Lilly and Novo struggled to keep up with the immense demand for their blockbuster meds.
In addition to increased demand, the shortfalls also owed to manufacturing delays and supply chain challenges.
While Mounjaro and Zepbound are back in stock, they’re still listed as unavailable on Lilly’s third-party provider Amazon Pharmacy.
XTALKS WEBINAR: Sarcopenia: Changes and Challenges in Body Composition with GLP-1 Receptor Agonist-based Weight Loss Therapies
Live and On-Demand: Friday, September 6, 2024, at 10am EDT (4pm CEST/EU-Central)
Register for this free webinar to learn about the latest clinical trial data on GLP-1 receptor agonist-based therapies and their impact on weight loss and body composition.
Lilly has been collaborating with the FDA to resolve supply issues, increase production and implement measures to stabilize the supply chain.
To tackle shortages, both Lilly and Novo have scaled up operations at current sites and are investing in new production facilities.
At the beginning of the month, Lilly CEO David Ricks told Bloomberg that he expected the shortage to end “very soon,” adding that “actually today or tomorrow we plan to exit that process.”
On a recent earnings call, Lilly announced that seven sites are expected to help alleviate the supply issues. Meanwhile, the company is gearing up for the launch of new manufacturing plants in North Carolina and Germany, which are slated to become operational by the end of the year and in 2027, respectively.
Lilly also put an extra $5.3 billion towards its 600-acre Indiana manufacturing complex in May this year, with the total investment now sitting at $9 billion. Lilly CEO David Ricks said the site was the largest outlay in US history for synthetic medicine active pharmaceutical ingredient (API) manufacturing.
Related: Oprah Hosts TV Special About GLP-1 Drugs for Weight Loss
Meanwhile, Novo said it is also increasing its manufacturing capacity and continuing to run its facilities 24 hours a day, seven days a week.
As part of expanding production capacity, the company will be acquiring three Catalent production facilities from Novo Holdings for an upfront payment of $11 billion, with the acquisition expected to be completed towards the end of 2024. In late 2023, Novo announced an approximately $9.5 billion investment in expanding and building new production sites in Denmark (estimated at $7 billion) and France (around $2.5 billion).
As supplies of Ozempic and Wegovy are restored, Doug Langa, Novo’s head of North American operations, said on a call that the company is seeing “more than double the number of prescriptions in the market compared to the beginning of the year” in the US.
Novo also reported an increase of 25 percent in sales in the first half of 2024, totaling 133.4 billion Danish kroner ($27.07 billion).
If you want your company to be featured on Xtalks.com, please email [email protected].











Join or login to leave a comment
JOIN LOGIN