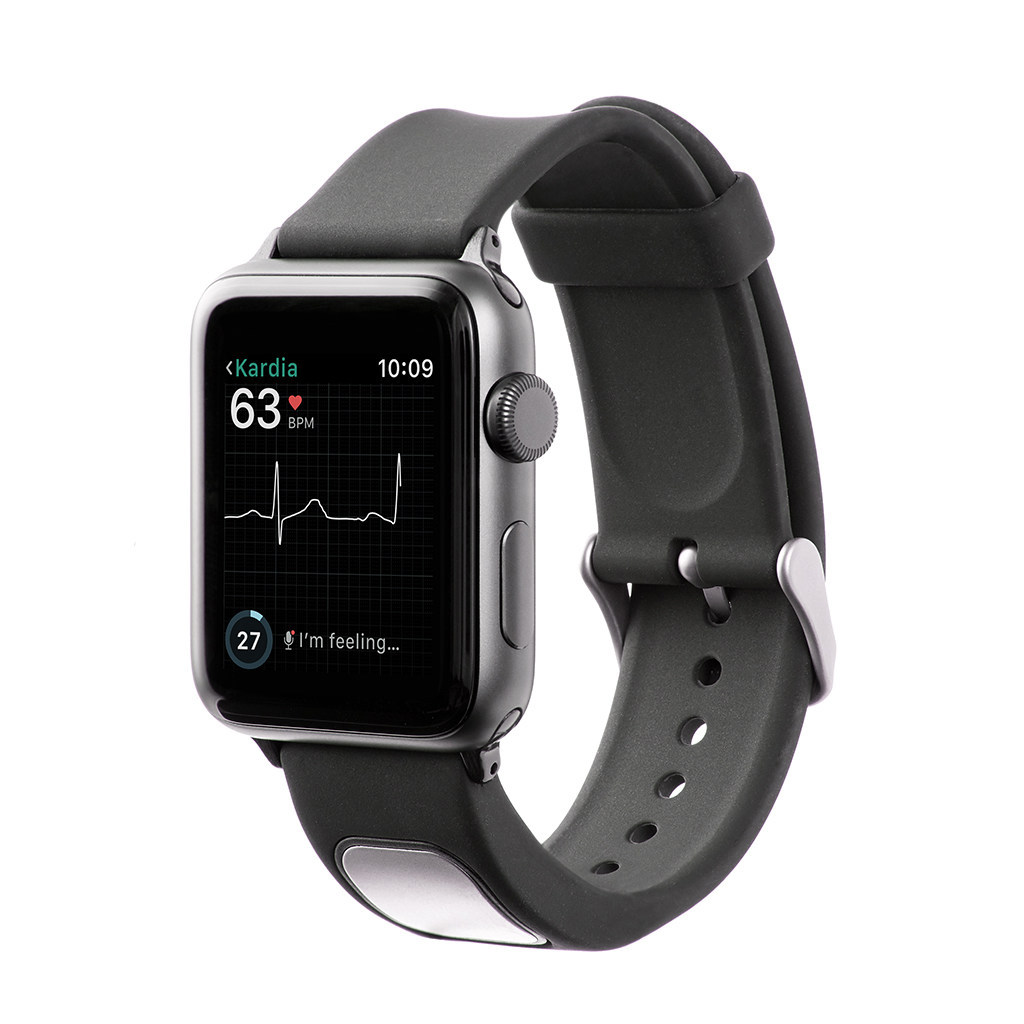
AliveCor’s KardiaBand has become the first medical device accessory for the Apple Watch to gain US Food and Drug Administration (FDA) approval. The device uses technology capable of collecting electrocardiogram (EKG) data and allowing the wearer to detect heart arrhythmias, such as atrial fibrillation (AFib).
It takes just 30 seconds for the KardiaBand to record an EKG, the results of which are displayed on the face of the Apply Watch. While the user can take these readings anytime, anywhere, the associated Kardia app includes a new feature known as SmartRhythm which prompts users to record an EKG at specific times.
An artificial intelligence-based system, the app compares heart rate and physical activity data collected by the Apple Watch to establish patterns. If SmartRhythm detects a change in heart rate that is inconsistent with the current level of physical activity, the device prompts the wearer to take an EKG and record the event using the KardiaBand.
“KardiaBand paired with SmartRhythm technology will be life-changing for people who are serious about heart health,” said Vic Gundotra, CEO, AliveCor. “These capabilities will allow people to easily and discreetly check their heart rhythms when they may be abnormal, capturing essential information to help doctors identify the issue and inform a clear path of care to help manage AFib, a leading cause of stroke, and other serious conditions.”
Atrial fibrillation is the most common form of heart arrhythmia, affecting over 30 million people globally. Twenty-five percent of adults over the age of 40 are at risk of developing atrial fibrillation, which has been identified as a common, and preventable, cause of stroke.
“This is a paradigm shift for cardiac care as well as an important advance in healthcare,” said Dr. Ronald P. Karlsberg, Board Certified Cardiologist and Clinical Professor of Medicine, Cedars Sinai Heart Institute and David Geffen School of Medicine UCLA. “Today, EKGs are available only in offices and hospitals, using complex equipment, and usually only after a life-threatening event, for example a stroke.
“With an EKG device on the wrist, AFib can be detected wherever the patient is, 24 hours a day. In randomized research trials, KardiaMobile, the first AliveCor EKG device, proved to be superior to routine care provided by physicians.”
The KardiaBand itself will be sold for $199, but users will need to subscribe to AliveCor’s service for $99 per year to keep a history of their EKG readings on the cloud. Using the app, EKG readings can also be forwarded to physicians or other healthcare providers via email, and AliveCor sends paper reports to its subscribers each month.










Join or login to leave a comment
JOIN LOGIN