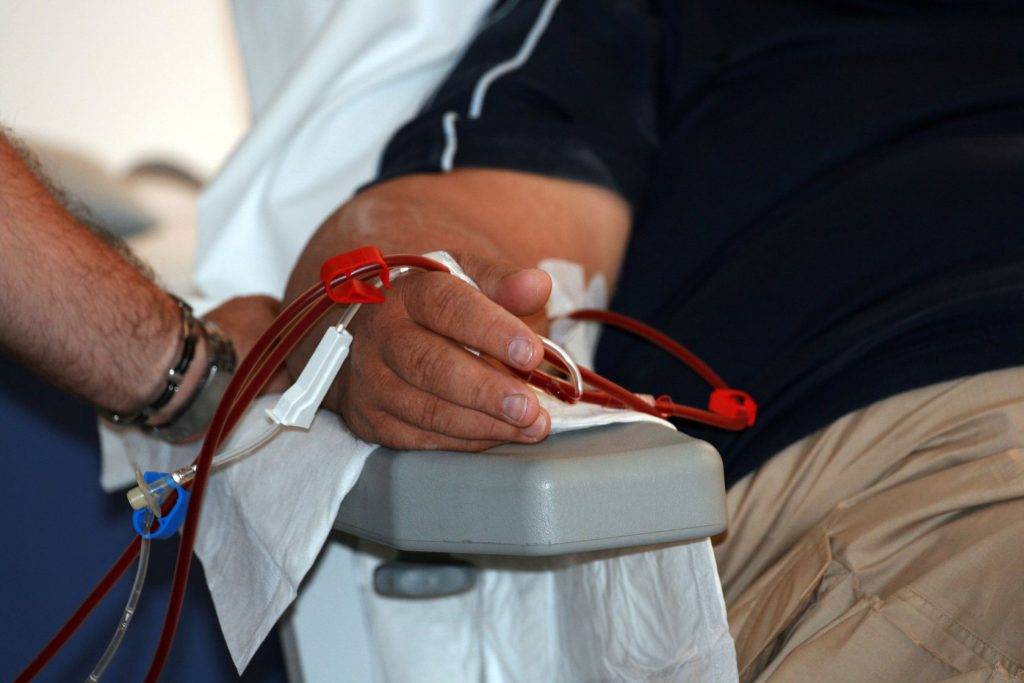
Patients in the end-stages of renal failure rely on hemodialysis to filter their blood, however this often requires them to make frequent visits to a local hospital or clinic. Now, the US Food and Drug Administration (FDA) has approved NxStage Medical’s System One for solo home hemodialysis, alleviating renal patients’ reliance on nurses and other care providers.
“We are thrilled with this milestone achievement,” said Todd Snell, SVP of QA, Regulatory & Clinical Affairs, NxStage. “Our interaction with the FDA first began at a patient preference workshop followed by regular dialogue throughout the pre-submission and submission processes. The solo home hemodialysis clearance makes NxStage the first company to formally conduct a patient preference study leading to a label expansion.”
While other home dialysis devices are available on the market, this is the first time the regulator has approved a system intended for solo use by the patient. The newly- expanded indication for the System One device will allow trained patients to perform their own dialysis at home.
NxStage Medical’s hemodialysis device was originally approved for home use in 2005 – an approval that was expanded to include nocturnal hemodialysis nearly 10 years later. Since its initial approval, thousands of patients have undergone millions of hemodialysis treatments in their homes, with the help of a care provider.
“Patients have been asking for an FDA-cleared solo option for years,” said Dr. Allan Collins, Chief Medical Officer, NxStage. “Many patients have been turned away from home hemodialysis simply because they did not have a care partner. The ability to train and treat solo provides a broader patient base with access to the clinical and quality of life benefits associated with home hemodialysis.”
Solo use of the System One hemodialysis unit has only been approved for daytime use. Patients who use the medical device at night will still require assistance from a care provider.
According to NxStage, patient training will be offered later this year, and in early 2018.










Join or login to leave a comment
JOIN LOGIN